Homo antecessor
Homo antecessor (Latin "pioneer man") is an extinct species of archaic human recorded in the Spanish Sierra de Atapuerca, a productive archaeological site, from 1.2 to 0.8 million years ago during the Early Pleistocene. Populations of this species may have been present elsewhere in Western Europe, and were among the first to colonise that region of the world, hence the name. The first fossils were found in the Gran Dolina cave in 1994, and the species was formally described in 1997 as the last common ancestor of modern humans and Neanderthals, supplanting the more conventional H. heidelbergensis in this position. H. antecessor has since been reinterpreted as an offshoot from the modern human line, although probably one branching off just before the modern human/Neanderthal split.
| Homo antecessor Temporal range: Early Pleistocene, | |
|---|---|
_y_mand%C3%ADbula_(parte_del_esqueleto_facial_ATD6-69)_del_Ni%C3%B1o_de_la_Gran_Dolina_(Hom%C3%ADnido_3)._Museo_Arqueol%C3%B3gico_Nacional_de_Espa%C3%B1a.jpg.webp) | |
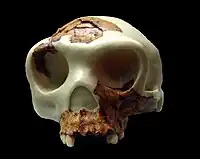
| The "Boy of Gran Dolina" fossils ATD6-15 (frontal bone) ATD6-69 (maxilla) Museo Arqueológico Nacional, Madrid | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Primates |
| Suborder: | Haplorhini |
| Infraorder: | Simiiformes |
| Family: | Hominidae |
| Subfamily: | Homininae |
| Tribe: | Hominini |
| Genus: | Homo |
| Species: | †H. antecessor |
| Binomial name | |
| †Homo antecessor Bermúdez de Castro et al., 1997 | |
Despite being so ancient, the face is unexpectedly similar to that of modern humans rather than other archaic humans—namely in its overall flatness as well as the curving of the cheekbone as it merges into the upper jaw—although these elements are known only from a juvenile specimen. Brain volume could have been 1,000 cc (61 cu in) or more, but no intact braincase has been discovered. For comparison, present-day modern humans average 1,270 cm3 for males and 1,130 cm3 for females. Stature estimates range from 162.3–186.8 cm (5 ft 4 in – 6 ft 2 in). H. antecessor may have been broad-chested and rather heavy, much like Neanderthals, although the limbs were proportionally long, a trait more frequent in tropical populations. The kneecaps are thin and have poorly developed tendon attachments. The feet indicate H. antecessor walked differently compared to modern humans.
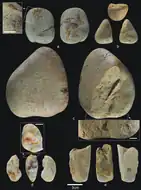
H. antecessor was predominantly manufacturing simple pebble and flake stone tools out of quartz and chert, although they used a variety of materials. This industry has some similarities with the more complex Acheulean, an industry which is characteristic of contemporary African and later European sites. Groups may have been dispatching hunting parties, which mainly targeted deer in their savannah and mixed woodland environment. Many of the H. antecessor specimens were cannibalised, perhaps as a cultural practice. There is no evidence they were using fire, and they similarly only inhabited inland Iberia during warm periods, presumably retreating to the coast otherwise.
Taxonomy
Research history
.jpg.webp)
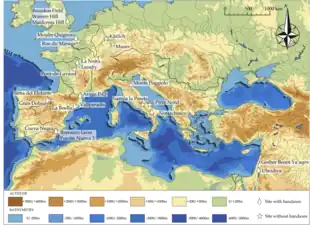
The Sierra de Atapuerca in northern Spain had long been known to be abundant in fossil remains. The Gran Dolina ("great sinkhole") was first explored for fossils by archaeologist Francisco Jordá Cerdá in a short field trip to the region in 1966, where he recovered a few animal fossils and stone tools. He lacked the resources and manpower to continue any further. In 1976, Spanish palaeontologist Trinidad Torres investigated the Gran Dolina for bear fossils (he recovered Ursus remains), but was advised by the Edelweiss Speleological Team to continue at the nearby Sima de los Huesos ("bone pit"). Here, in addition to a wealth of bear fossils, he also recovered archaic human fossils, which prompted a massive exploration of the Sierra de Atapuerca, at first headed by Spanish palaeontologist Emiliano Aguirre but quickly taken over by José María Bermúdez de Castro, Eudald Carbonell, and Juan Luis Arsuaga. They restarted excavation of the Gran Dolina in 1992, and found archaic human remains two years later, which in 1997 they formally described as a new species, Homo antecessor.[1] The holotype is specimen ATD6-5, a right mandibular fragment retaining the molars and recovered with some isolated teeth. In their original description Castro and colleagues posited that the species was the first human to colonise Europe, hence the name antecessor (Latin for "explorer", "pioneer", or "early settler").[2]
The 25 m (82 ft) of Pleistocene sediments at the Gran Dolina are divided into eleven units, TD1 to TD11 ("trinchera dolina" or "sinkhole trench"). H. antecessor was recovered from TD6, which has consequently become the most well researched unit of the site.[3] In the first field seasons from 1994–1995, the dig team excavated a small test pit (to see if the unit warranted further investigation) in the southeast section measuring 6 m2 (65 sq ft).[3] Human fossils were discovered first by Aurora Martín Nájera; the 30 cm (12 in) layer they were found in is nicknamed the "Aurora Stratum" after her.[4] A 13 m2 (140 sq ft) triangular section was excavated in the central section starting in the early 2000s.[3] Human fossils were also found in the northern section. In sum, about 170 H. antecessor specimens were recovered.[3] The best preserved are ATD6-15 and ATD6-69 (possibly belonging to the same individual) that most clearly elucidate facial anatomy.[2] Subsequent field seasons have yielded about sixty more specimens.[1] The discovered parts of the H. antecessor skeleton are: elements of the face, clavicle, forearm, digits, knees, and a few vertebrae and ribs.[5]
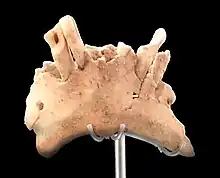
In 2007 a mandibular fragment with some teeth, ATE9-1, provisionally assigned to H. antecessor by Carbonell, was recovered from the nearby Sima del Elefante ("elephant pit") in unit TE9 ("trinchera elefante"), belonging to a 20–25-year-old individual. The site additionally yielded stone flakes and evidence of butchery.[6] In 2011, after providing a much more in depth analysis of the Sima del Elefante material, Castro and colleagues were unsure of the species classification, opting to leave it at Homo sp. (making no opinion on species designation) pending further discoveries.[7]
The stone tool assemblage at the Gran Dolina is broadly similar to several other contemporary ones across Western Europe, which may represent the work of the same species, although this is unconfirmable because many of these sites have not produced human fossils.[2] In 2014 fifty footprints dating to between 1.2 million and 800,000 years ago were discovered in Happisburgh, England, which could potentially be attributed to an H. antecessor group given it is the only human species identified during that time in Western Europe.[8]
Age and taphonomy
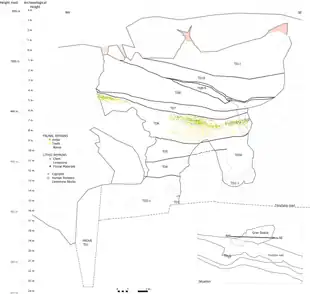
The 2003 to 2007 excavations revealed a much more intricate stratigraphy than previously thought, and TD6 was divided into three subunits spanning thirteen layers and nine sedimentary facies (bodies of rock distinctive from adjacent bodies). Human presence is recorded in subunits 1 and 2, and in facies A, D1, and F. Randomly orientated scattered bones were deposited in Facies D1 of layer TD6.2.2 (TD6 subunit 2, layer 2) and Facies F of layers TD6.2.2 and TD6.2.3, but in Facies D they seem to have been conspicuously clumped into the northwest area. This might indicate they were dragged into the cave via a debris flow. As for Facies F, which contains the most human remains, they may have been deposited by a low energy debris flow (consistent with floodplain behaviour) from the main entrance to the northwest, as well as a stronger debris flow from another entrance to the south. Fluvially deposited fossils (dragged in by a stream of water) were also recovered from Facies A in layers TD6.2.2, TD6.2.1 and TD6.1.2, indicated by limestone gravel within the size range of the remains. Thus, H. antecessor may not have inhabited the cave, although was at least active nearby. Only 5.6% of the fossils bear any evidence of weathering from open air, roots, and soil, which could mean they were deposited deep into the cave relatively soon after death.[3]
Human occupation seems to have occurred in waves corresponding to timespans featuring a warm, humid savannah habitat (although riversides likely supported woodlands). These conditions were only present during transitions from cool glacial to warm interglacial periods, after the climate warmed and before the forests could expand to dominate the landscape.[10] The dating attempts of H. antecessor remains are:
- In 1999 two ungulate teeth from TD6 were dated using uranium–thorium dating to 794 to 668 thousand years ago, and further constrained palaeomagnetically to before 780,000 years ago.[11]
- In 2008 TE9 of the Sima del Elefante was constrained to 1.2–1.1 million years ago using palaeomagnetism and cosmogenic dating.[6]
- In 2013 TD6 was dated to about 930 to 780 thousand years ago using palaeomagnetism, in addition to uranium–thorium and electron spin resonance dating (ESR) on more teeth.[12]
- In 2018 ESR dating of the H. antecessor specimen ATD6-92 resulted in an age of 949 to 624 thousand years ago, further constrained palaeomagnetically to before 772,000 years ago.[13]
- In 2022 ESR and single grain thermally transferred optically stimulated luminescence (SG TT-OSL) dated the opening of the Gran Dolina to roughly 900,000 years ago, and the sediments from TD4 to TD6 to between 890,000 to 770,000 years ago. These three units were probably deposited within a period of less than 100,000 years.[14]
Until 2013 with the discovery of the 1.4 million-year-old infant tooth from Barranco León, Orce, Spain, these were the oldest human fossils known from Europe,[15] although human activity on the continent stretches back as early as 1.6 million years ago in Eastern Europe and Spain indicated by stone tools.[16]
Classification

The face of H. antecessor is unexpectedly similar to that of modern humans compared to other archaic groups, so in their original description, Castro and colleagues classified it as the last common ancestor of modern humans and Neanderthals, supplanting H. heidelbergensis in this capacity.[2][5][1] The facial anatomy came under close scrutiny in subsequent years.[1]
In 2001 French palaeoanthropologist Jean-Jacques Hublin postulated that the Gran Dolina remains and the contemporaneous Tighennif remains from Algeria (usually classified as Homo ergaster [=? Homo erectus], originally "Atlantanthropus mauritanicus"[lower-alpha 1]) represent the same population, because fourteen of the fifteen dental features Castro and colleagues listed for H. antecessor have also been identified in the Middle Pleistocene of North Africa; this would mean H. antecessor is a junior synonym of "Homo mauritanicus", i. e., the Gran Dolina and Tighennif humans should be classified into the latter.[20] In 2007 Castro and colleagues studied the fossils, and found the Tighennif remains to be much larger than H. antecessor and dentally similar to other African populations. Nonetheless, they still recommended reviving mauritanicus to house all Early Pleistocene North African specimens as "H. ergaster mauritanicus".[21]
In 2007 primatologist Esteban Sarmiento and colleagues questioned the legitimacy of H. antecessor as a separate species because much of the skull anatomy is unknown; H. heidelbergensis is known from roughly the same time and region; and because the type specimen was a child (the supposedly characteristic features could have disappeared with maturity.) Such restructuring of the face, they argued, can also be caused by regional climatic adaptation rather than speciation.[22] In 2009 American palaeoanthropologist Richard Klein stated he was skeptical that H. antecessor was ancestral to H. heidelbergensis, interpreting H. antecessor as "an offshoot of H. ergaster [from Africa] that disappeared after a failed attempt to colonize southern Europe".[23] Similarly, in 2012, British physical anthropologist Chris Stringer considered H. antecessor and H. heidelbergensis to be two different lineages rather than them having an ancestor/descendant relationship.[17] In 2013, anthropologist Sarah Freidline and colleagues suggested the modern humanlike face evolved independently several times among Homo.[24] In 2017 Castro and colleagues conceded that H. antecessor may or may not be a modern human ancestor, although if it was not then it probably split quite shortly before the modern human/Neanderthal split.[25] In 2020 Dutch molecular palaeoanthropologist Frido Welker and colleagues concluded H. antecessor is not a modern human ancestor by analysing ancient proteins collected from the tooth ATD6-92.[26]
Anatomy
Skull
The facial anatomy of H. antecessor is predominantly known from the 10–11.5-year-old H. antecessor child ATD6-69, as the few other facial specimens are fragmentary. ATD6-69 is strikingly similar to modern humans (as well as East Asian Middle Pleistocene archaic humans) as opposed to West Eurasian or African Middle Pleistocene archaic humans including Neanderthals. The most notable traits are a completely flat face and a curved zygomaticoalveolar crest (the bar of bone connecting the cheek to the part of the maxilla that holds the teeth). In 2013 anthropologist Sarah Freidline and colleagues statistically determined that these features would not disappear with maturity. H. antecessor suggests the modern human face evolved and disappeared multiple times in the past, which is not unlikely as facial anatomy is strongly influenced by diet and thus the environment.[24] The nasal bones are like those of modern humans.[2] The mandible (lower jaw) is quite gracile unlike most other archaic humans. It exhibits several archaic features, but the shape of the mandibular notch is modern humanlike, and the alveolar part (adjacent to the teeth) is completely vertical as in modern humans. Like many Neanderthals, the medial pterygoid tubercle is large. Unlike most Neanderthals, there is no retromolar space (a large gap between the last molar and the end of the body of the mandible).[25]
The upper incisors are shovel-shaped (the lingual, or tongue, side is distinctly concave), a feature characteristic of other Eurasian human populations, including modern. The canines bear the cingulum (a protuberance toward the base) and the essential ridge (toward the midline) like more derived species, but retain the cuspules (small bumps) near the tip and bordering incisor like more archaic species. The upper premolar crowns are rather derived, being nearly symmetrical and bearing a lingual cusp (on the tongue side), and a cingulum and longitudinal grooves on the cheekward side. The upper molars feature several traits typically seen in Neanderthals. The mandibular teeth, on the other hand, are quite archaic. The P3 (the first lower premolar) has a strongly asymmetrical crown and complex tooth root system. P3 is smaller than P4 like in more derived species, but like other early Homo, M1 (the first lower molar) is smaller than M2 and the cusps of the molar crowns make a Y shape. The distribution of enamel is Neanderthal-like, with thicker layers at the periphery than at the cusps.[25] Based on two canine teeth (ATD6- 69 and ATD6-13), the thickness of the enamel and the proportion of the tooth covered by the gums vary to the same degree as for males and females of modern humans and many other apes, so this may be due to sexual dimorphism, with females having smaller teeth, relatively thicker enamel, and smaller proportion of gum coverage.[27]
The parietal bones (each being one side of the back part of the top of the skull) are flattened, and conjoin at a peak at the midline. This "tent-like" profile is also exhibited in more archaic African H. ergaster and Asian H. erectus. Like H. ergaster, the temporal styloid process just below the ear is fused to the base of the skull. The brow ridges are prominent. The upper margin of the squamous part of temporal bones (on the side of the skull) is convex, like in more derived species.[25] The brain volume of ATD6-15, perhaps belonging to an 11-year-old, may have been 1,000 cc (61 cu in) or more based on frontal bone measurements.[lower-alpha 2][2] For comparison, present-day modern humans average 1,270 cm3 for males and 1,130 cm3 for females, with a standard deviation of roughly 115 and 100 cm3.[28]
Torso
The notably large adult clavicle specimen ATD6-50, assumed male based on absolute size, was estimated to have stood 162.3–186.8 cm (5 ft 4 in – 6 ft 2 in), mean of 174.5 cm (5 ft 9 in), based on the correlation among modern Indian people between clavicle length and stature. An adult radius (a forearm bone), ATD6-43, which could be male based on absolute size or female based on gracility, was estimated to have belonged to a 172.5 cm (5 ft 8 in) tall individual based on the average of equations among several modern populations relating radial length to stature. Based on metatarsal (foot bone) length, a male is estimated to have stood 173 cm (5 ft 8 in) and a female 168.9 cm (5 ft 6 in). These are all rather similar values. For comparison, Western European Neanderthal estimates average 165.3 cm (5 ft 5 in), and early European modern humans 178.4 cm (5 ft 10 in).[5] The ankle joint is adapted for handling high stress, which may indicate a heavy, robust body plan, much like Neanderthals.[29] Based on the relationship between human footprint length and body size, twelve Happisburgh prints that are preserved well enough to measure are consistent with individuals ranging from 93 to 173 cm (3 ft 1 in to 5 ft 8 in) in stature, which may mean some of the trackmakers were children. By this logic, the three biggest footprints—equating to statures of 160 cm (5 ft 3 in), 163 cm (5 ft 4 in), and 173 cm (5 ft 8 in)—ranged from 48 to 53 kg (106 to 117 lb) in weight. Stature estimates for H. antecessor, H. heidelbergensis, and Neanderthals are roughly consistent with each other.[8]

Two atlases (the first neck vertebra) are known, which is exceptional as this bone is rarely discovered for archaic humans. They are indistinguishable from those of modern humans. For the axis (the second neck vertebra), the angle of the spinous process (jutting out from the vertebra) is about 19°, comparable with Neanderthals and modern humans, diverging from H. ergaster with a low angle of about 8°. The vertebral foramen (that houses the spinal cord) is on the narrow side compared to modern humans. The spine as a whole otherwise aligns with modern humans.[5]
There is one known (and incomplete) clavicle, ATD6-50, which is thick compared to those of modern humans. This may indicate H. antecessor had long and flattish (platycleidic) clavicles like other archaic humans. This would point to a broad chest. The proximal curvature (twisting of the bone on the side nearest the neck) in front view is on par with that of Neanderthals, but the distal curvature (on the shoulder side) is much more pronounced. The sternum is narrow. The acromion (that extends over the shoulder joint) is small compared to those of modern humans.[5] The shoulder blade is similar to all Homo with a typical human body plan, indicating H. antecessor was not as skilled a climber as non-human apes or pre-erectus species, but was capable of efficiently launching projectiles such as stones or spears.[30]
Limbs
The incomplete radius, ATD6-43, was estimated to have measured 257 mm (10.1 in). It is oddly long and straight for someone from so far north, reminiscent of the proportions seen in early modern humans and many people from tropical populations. This could be explained as retention of the ancestral long limbed tropical form, as opposed to Neanderthals who evolved shorter limbs. This could also indicate a high brachial index (radial to humeral length ratio). Compared to more recent human species, the cross section of the radial shaft is rather round and gracile throughout its length. Like archaic humans, the radial neck (near the elbow) is long, giving more leverage to the biceps brachii. Like modern humans and H. heidelbergensis, but unlike Neanderthals and more archaic hominins, the radial tuberosity (a bony knob jutting out just below the radial neck) is anteriorly placed (toward the front side when the arm is facing out).[5]
Like those of other archaic humans, the femur features a developed trochanteric fossa and posterior crest. These traits are highly variable among modern human populations. The two known kneecaps, ATD6-22 and ATD6-56, are subrectangular in shape as opposed to the more common subtriangular, although rather narrow like those of modern humans. They are quite small and thin, falling at the lower end for modern human females. The apex of the kneecap (the area that does not join to another bone) is not well developed, leaving little attachment for the patellar tendon. The medial (toward the midline) facet and lateral (toward the sides) facet for the knee joint are roughly the same size as each other in ATD6-56 and the medial is larger in ATD6-22, whereas the lateral is commonly larger in modern humans. The lateral facet encroaches onto a straight flat area as opposed to being limited to a defined vastus notch, an infrequent condition among any human species.[5]
The phalanges and metatarsals of the foot are comparable to those of later humans, but the big toe bone is rather robust, which could be related to how H. antecessor pushed off the ground. The ankle bone (talus) is exceptionally long and high as well as the facet where it connects with the leg (the trochlea), which may be related to how H. antecessor walked. The long trochlea caused a short neck of the talus, which bridges the head of the talus connecting to the toes, and the body of the talus connecting to the leg. This somewhat converges with the condition exhibited in Neanderthals, which is generally explained as a response to a heavy and robust body, to alleviate the consequently higher stress to the articular cartilage in the ankle joint. This would also have permitted greater flexion.[29]
Growth rate
.jpg.webp)
Natural History Museum, London
In 2010 Castro and colleagues estimated that ATD6-112, represented by a permanent upper and lower first molar, died between 5.3 and 6.6 years of age based on the tooth formation rates in chimpanzees (lower estimate) and modern humans (upper). The molars are hardly worn at all, which means the individual died soon after the tooth erupted, and that first molar eruption occurred at roughly this age. The age is within the range of variation of modern humans, and this developmental landmark can debatably be correlated with life history. If the relation is true, H. antecessor had a prolonged childhood, a characteristic of modern humans in which significant cognitive development takes place.[31]
Pathology
The partial face ATD6-69 has an ectopic M3 (upper left third molar), where it erupted improperly, and this caused the impaction of M2, where it was blocked from erupting at all. Although impaction of M3 is rather common in modern humans, as high as fifty percent in some populations, impaction of M2 is rare, as little as 0.08 to 2.3%. Impaction can lead to secondary lesions, such as dental cavities, root resorption, keratocysts and dentigerous cysts.[32]
The mandible ATE9-1 exhibits severe dental attrition and abrasion of the tooth crowns and bone resorption at the root, so much so that the root canals (the sensitive interior) of the canines are exposed. The trauma is consistent with gum disease due to overloading the teeth, such as by using the mouth as a third hand to carry around items. A similar condition was also reported for the later Sima de los Huesos remains also at the Sierra de Atapuerca site.[33]
The left knee bone ATD6-56 has a 4.7 mm × 15 mm (0.19 in × 0.59 in) height x breadth osteophyte (bone spur) on the inferior (lower) margin. Osteophytes normally form as a response to stress due to osteoarthritis, which can result from old age or improper loading of the joint as a consequence of bone misalignment or ligament laxity. In the case of ATD6-56, improper loading was likely the causal factor. Frequent squatting and kneeling can lead to this condition, but if the right knee bone ATD6-22 (that has no such trauma) belongs to the same individual, then this is unlikely to be the reason. If so, the lesion was caused by a local trauma, such as strain on the soft tissue around the joint due to high intensity activity, or a fracture of the left femur and/or tibia (that is unconfirmable since neither bone is associated with this individual).[34]
The right fourth metatarsal ATD6-124 has a 25.8 mm × 8 mm (1.02 in × 0.31 in) length x width lesion on the medial (toward the midline of the bone) side consistent with a march fracture. This condition is most often encountered by soldiers, long distance runners, and potentially flatfooted people whose foot bones failed under repeated, high intensity activity. Later Neanderthals would evolve a much more robust lower skeleton possibly to withstand such taxing movement across uneven terrain. Although only one other example of the condition has been identified (at Sima de los Huesos) among archaic humans, march fractures were probably a common injury for them given that the healed fracture leaves no visible mark, as well as their presumed high intensity lifestyle.[35]
Culture
Technology
H. antecessor was producing simple stone tools at Gran Dolina. This industry is found elsewhere in Early Pleistocene Spain—notably in Barranc de la Boella and the nearby Galería—distinguished by the preparation and sharpening of cores before flaking, the presence of (crude) bifaces, and some degree of standardisation of tool types. This bears some resemblance to the much more complex Acheulean industry, characteristic of African and later European sites.[9] The earliest evidence of typical Acheulean toolsets comes from Africa 1.75 million years ago, but the typical Acheulean toolset pops up in Western Europe nearly a million years later. It is debated if these early European sites evolved into the European Acheulean industry independently from African counterparts, or if the Acheulean was brought up from Africa and diffused across Europe. In 2020 French anthropologist Marie-Hélène Moncel argued the appearance of typical Achuelean bifaces 700,000 years ago in Europe was too sudden to be the result of completely independent evolution from local technologies, so there must have been influence from Africa.[36] Wearing on the TD6 stone tools is consistent with repeated abrasion against flesh, so they were probably used as butchering implements.[9]
TD6.3
In the lower part of TD6.3 (TD6 subunit 3), 84 stone tools were recovered, predominantly small, unmodified quartzite pebbles with percussive damage—probably inflicted from pounding items such as bone—as opposed to manufacturing more specialised implements.[9]
Although 41% of the section's assemblage consists of flakes, they are rather crude and large—averaging 38 mm × 30 mm × 11 mm (1.50 in × 1.18 in × 0.43 in)—either resulting from rudimentary knapping (stoneworking) skills or difficulty working such poor quality materials. They made use of the unipolar longitudinal method, flaking off only one side of a core, probably to compensate for the lack of preplanning, opting to knap irregularly shaped and thus poorer quality pebbles.[9]
TD6.2
Most of the stone tools resided in the lower (older) half of TD6.2, with 831 stone tools. The knappers made use of a much more diverse array of materials (although most commonly chert), which indicates they were moving farther out in search of better raw materials. The Sierra de Atapuerca features an abundance and diversity of mineral outcroppings suitable for stone tool manufacturing, in addition to chert and quartz namely quartzite, sandstone, and limestone, which could all be collected within only 3 km (1.9 mi) of the Gran Dolina.[9]
They produced far fewer pebbles and spent more time knapping off flakes, but they were not particularly economic with their materials, and about half of the cores could have produced more flakes. They additionally modified irregular blanks into more workable shapes before flaking off pieces. This preplanning allowed them to use other techniques: the centripetal method (flaking off only the edges of the core) and the bipolar method (laying the core on an anvil and slamming it with a hammerstone). There are 62 flakes measuring below 20 mm (0.79 in) in height, and 28 above 60 mm (2.4 in). There are three conspicuously higher quality flakes, thinner and longer than the others, which may have been produced by the same person. There are also retouched tools: notches, spines, denticulates, points, scrapers, and a single chopper. These small retouched tools are rare in the European Early Pleistocene.[9]
TD6.1
TD6.1 yielded 124 stone tools, but they are badly preserved as the area was also used by hyenas as a latrine, the urine corroding the area. The layer lacks pebbles and cores, and 44 of the stone tools are indeterminate. Flakes are much smaller with an average of 28 mm × 27 mm × 11 mm (1.10 in × 1.06 in × 0.43 in), with ten measuring below 20 mm (0.79 in), and only three exceeding 60 mm (2.4 in).[9]
They seem to have been using the same methods as the people who manufactured the TD6.2 tools. They were only retouching larger flakes, the fourteen such tools averaging 35 mm × 26 mm × 14 mm (1.38 in × 1.02 in × 0.55 in): one marginally retouched flake, one notch, three spines, seven denticulate sidescrapers, and one denticulate point.[9]
Fire and palaeoclimate
Only a few charcoal particles have been collected from TD6, which probably originated from a fire well outside the cave. There is no evidence of any fire use or burnt bones (cooking) in the occupation sequences of the Gran Dolina. In other parts of the world, reliable evidence of fire usage does not surface in the archaeological record until roughly 400,000 years ago.[37] In 2016, small mammal bones burned in fires exceeding 600 °C (1,112 °F) were identified from 780- to 980-thousand-year-old deposits at Cueva Negra in southern Spain, which potentially could have come from a human source as such a high temperature is usually (though not always) recorded in campfires as opposed to natural bushfires.[38]
Instead of using fire, these early Europeans probably physiologically withstood the cold, such as by eating a high protein diet to support a heightened metabolism.[37] Despite glacial cycles, the climate was probably similar or a few degrees warmer compared to that of today's, with the coldest average temperature reaching 2 °C (36 °F) sometime in December and January, and the hottest in July and August 18 °C (64 °F). Freezing temperatures could have been reached from November to March, but the presence of olive and oak suggests subfreezing was an infrequent occurrence.[22] TE9 similarly indicates a generally warm climate.[6] The Happisburgh footprints were lain in estuarine mudflats with open forests dominated by pine, spruce, birch, and in wetter areas alder, with patches of heath and grasslands; the vegetation is consistent with the cooler beginning or end of an interglacial.[8]
H. antecessor probably migrated from the Mediterranean shore into inland Iberia when colder glacial periods were transitioning to warmer interglacials, and warm grasslands dominated, vacating the region at any other time. They may have followed water bodies while migrating, in the case of Sierra de Atapuerca, most likely the Ebro River.[10]
Food
The fossils of sixteen animal species were recovered[39] randomly mixed[4] with the H. antecessor material at the Gran Dolina, including the extinct bush-antlered deer, the extinct species of fallow deer Dama vallonetensi, the extinct subspecies of red deer Cervus elaphus acoronatus, the extinct bison Bison voigstedtensi, the extinct rhino Stephanorhinus etruscus, the extinct horse Equus stenonis, the extinct fox Vulpes praeglacialis, the extinct bear Ursus dolinensis, the extinct wolf Canis mosbachensis, the spotted hyena, the wild boar, and undetermined species of mammoth, monkey, and lynx. Some specimens of the former eight species and the monkey exhibit cut marks consistent with butchery, with about 13% of all Gran Dolina remains bearing some evidence of human modification. Deer are the most commonly butchered animal, with 106 specimens. The inhabitants seem to have carried carcasses back whole when feasible, and only the limbs and skulls of larger quarries. This indicates the Gran Dolina H. antecessor were dispatching hunting parties who killed and hauled back prey to share with the entire group rather than each individual foraging entirely for themselves, which evinces social cooperation and division of labour. Less than 5% of all the remains retain animal carnivore damage, in two instances toothmarks overlapping cutmarks from an unidentified animal, which could indicate animals were sometimes scavenging H. antecessor leftovers.[39]

The Sima del Elefante site records the fallow deer, the bush-antlered deer, rhinos, E. stenonis, C. mosbachensis, U. dolinensis, the extinct big cat Panthera gombaszoegensis, the extinct lynx Lynx issiodorensis, the extinct fox Vulpes alopecoides, several rats, shrews, and rabbits, and undetermined species of macaques, boar, bison, and beaver. The large mammals are most commonly represented by long bones, a few of which are cracked open, presumably to access the bone marrow. Some others bear evidence of percussion and defleshing.[6] They were also butchering Hermann's tortoise, an easily obtainable source of meat considering how slowly tortoises move.[40]
The cool and humid montane environment encouraged the growth of olive, mastic, beech, hazelnut, and chestnut trees, which H. antecessor may have used as food sources, although they become more common in TD7 and TD8 as the interglacial progresses and the environment becomes wetter. In the H. antecessor unit TD6, pollen predominantly derives from juniper and oak. Trees probably grew along rivers and streams, while the rest of the hills and ridges were dominated by grasses.[22] The TD6 individuals also seem to have been consuming hackberries, which in historical times have been used for their medicinal properties more than satiating hunger because these berries provide very little flesh.[41]
There is no evidence H. antecessor could wield fire and cook, and similarly the wearing on the molars indicates the more frequent consumption of grittier and more mechanically challenging foods than later European species, such as raw rather than cooked meat and underground storage organs.[42]
Cannibalism
Eighty young adult and child H. antecessor specimens from the Gran Dolina exhibit cut marks and fracturing indicative of cannibalism,[5][39] and H. antecessor is the second-most common species bearing evidence of butchering.[39] Human bodies were efficiently utilised, and may be the reason why most bones are smashed or otherwise badly damaged. There are no complete skulls, elements from the face and back of the skull are usually percussed, and the muscle attachments on the face and the base of the skull were cut off. The intense modification of the face was probably to access the brain. The crown of the head was probably struck, resulting in the impact scars on the teeth at the gum line. Several skull fragments exhibit peeling.[4]
The ribs also bear cut marks along the muscle attachments consistent with defleshing, and ATD6-39 has cuts along the length of the rib, which may be related to disembowelment. The nape muscles were sliced off, and the head and neck were probably detached from the body. The vertebrae were often cut, peeled, and percussed. The muscles on all of the clavicles were sawed off to disconnect the shoulder. One radius, ATD6-43, was cut up and peeled. The femur was shattered, probably to extract the bone marrow. The hands and feet variably exhibit percussion, cutting, or peeling, likely a result of dismemberment.[4]
In sum, mainly the meatier areas were prepared, and the rest discarded. This suggests they were butchering humans for nutritional purposes, but the face generally exhibits significantly more cutmarks than the faces of animals. When this is seen in prehistoric modern human specimens, it is typically interpreted as evidence of exocannibalism, a form of ritual cannibalism where one eats someone from beyond their social group, such as an enemy from a neighbouring tribe. But, when overviewing the evidence of H. antecessor cannibalism in 1999, Spanish palaeontologist Yolanda Fernandez-Jalvo and colleagues instead ascribed the relative abundance of facial cut marks in the H. antecessor sample to the strongly contrasting structure of the muscle attachments between humans and typical animal prey items (that is, defleshing the human face simply required more cuts, or the butcherers were less familiar with defleshing humans).[4]
Nonetheless, the assemblage had a lack of older individuals, composed entirely of young adults and juveniles. In 2010 Carbonell hypothesised that they were practising exocannibalism and hunting down neighbouring tribesmen.[43] In 2019, Spanish palaeoanthropologist Jesús Rodríguez and colleagues argued that — considering the high youth mortality rates in modern hunter-gatherer groups – the demographic is better explained as consuming fellow tribesmen (already dead from natural causes, war, or an accident), possibly simply to avoid wasting food.[44]
See also
Notes
- The Tighennif remains were classified by French vertebrate paleontologist Camille Arambourg as Atlantanthropus mauritanicus in 1955.[18] This name is usually sunk into H. erectus, but many authors choose to distinguish Asian and African populations on a species level, with the latter classified into H. ergaster which was named in 1975. This is somewhat problematic as several African specimens sunk into H. ergaster were already given unique species designations which should take priority, including H. mauritanicus.[19]
- The frontal breadth (the length of the frontal bone) and the bistephanic breadth (the length between each stephanion) of ATD6-15 are respectively 95–100 mm (3.7–3.9 in) and 100 mm (3.9 in), which are substantially longer than what is measured in the H. erectus specimens KNM ER 3733, KNM ER 3883, Sangiran 2, or Trinil 2, which each have an estimated brain volume of less than 1,000 cc (61 cu in).[2]
References
- de Castro, J.-M. B. (2015). "Homo antecessor: The state of the art eighteen years later". Quaternary International. 433: 22–31. doi:10.1016/j.quaint.2015.03.049.
... a speciation event could have occurred in Africa/Western Eurasia, originating a new Homo clade [...] Homo antecessor [...] could be a side branch of this clade placed at the westernmost region of the Eurasian continent.
- Bermudez de Castro, J.M.; Arsuaga, J.L.; Carbonell, E.; Rosas, A.; Martinez, I.; Mosquera, M. (1997). "A hominid from the Lower Pleistocene of Atapuerca, Spain: possible ancestor to Neandertals and modern humans". Science. 276 (5317): 1392–1395. doi:10.1126/science.276.5317.1392. PMID 9162001. S2CID 31088294.
- Campaña, I.; Pérez-González, A.; Benito-Calvo, A.; Rosell, J.; Blasco, R.; de Castro, J. M. B.; Carbonell, E.; Arsuaga, J. L. (2016). "New interpretation of the Gran Dolina-TD6 bearing Homo antecessor deposits through sedimentological analysis". Scientific Reports. 6 (34799): 34799. Bibcode:2016NatSR...634799C. doi:10.1038/srep34799. PMC 5054435. PMID 27713562.
- Fernández-Jalvo, Y.; Díez, J. C.; Cáceres, I.; Rosell, J. (1999). "Human cannibalism in the Early Pleistocene of Europe (Gran Dolina, Sierra de Atapuerca, Burgos, Spain)". Journal of Human Evolution. 37 (34): 591–622. doi:10.1006/jhev.1999.0324. PMID 10497001. S2CID 25096156.
- Carretero, J. M.; Lorenzo, C.; Arsuaga, J. L. (1999). "Axial and appendicular skeleton of Homo antecessor". Journal of Human Evolution. 37 (3–4): 459–499. doi:10.1006/jhev.1999.0342. PMID 10496997.
- Carbonell, E. (2008). "The first hominin of Europe". Nature. 452 (7186): 465–469. Bibcode:2008Natur.452..465C. doi:10.1038/nature06815. hdl:2027.42/62855. PMID 18368116. S2CID 4401629.
- de Castro, J. M. B.; Martinón-Torres, M.; Gómez-Robles, A.; Prado-Simón, L.; Martín-Francésa, L.; Lapresa, M.; Olejniczaka, A.; Carbonell, E. (2011). "Early Pleistocene human mandible from Sima del Elefante (TE) cave site in Sierra de Atapuerca (Spain): A comparative morphological study". Journal of Human Evolution. 61 (1): 12–25. doi:10.1016/j.jhevol.2011.03.005. PMID 21531443.
- Ashton, N.; Lewis, S.G.; De Groote, I.; Duffy, S.M.; Bates, M.; Bates, R.; Hoare, P.; Lewis, M.; Parfitt, S. A.; Peglar, S.; Williams, W.; Stringer, C. (2014). "Hominin footprints from Early Pleistocene deposits at Happisburgh, UK". PLoS One. 9 (2): e88329. Bibcode:2014PLoSO...988329A. doi:10.1371/journal.pone.0088329. PMC 3917592. PMID 24516637.
- Mosquera, M.; Ollé, A.; Rodríguez-Álvarez, X. P.; Carbonell, E. (2018). "Shedding light on the Early Pleistocene of TD6 (Gran Dolina, Atapuerca, Spain): The technological sequence and occupational inferences". PLoS One. 13 (1): 0190889. Bibcode:2018PLoSO..1390889M. doi:10.1371/journal.pone.0190889. PMC 5784927. PMID 29370188.
- Blain, H.-L.; Cuenca-Bescós, G.; Burjachs, F.; López-García, J. M.; Lozano-Fernandéz, I.; Rosell, J. (2013). "Early Pleistocene palaeoenvironments at the time of the Homo antecessor settlement in the Gran Dolina cave (Atapuerca, Spain)". Journal of Quaternary Science. 28 (3): 311–319. Bibcode:2013JQS....28..311B. doi:10.1002/jqs.2622. S2CID 129402115.
- Falguères, C.; Bahain, J.; Yokoyama, Y.; Arsuaga, J.; Bermudez de Castro, J.; Carbonell, E.; Bischoff, J.; Dolo, J. (1999). "Earliest humans in Europe: the age of TD6 Gran Dolina, Atapuerca, Spain" (PDF). Journal of Human Evolution. 37 (3–4): 343–352. doi:10.1006/jhev.1999.0326. PMID 10496991.
- Parés, J.M.; Arnold, L.; Duval, M.; Demuro, M.; Pérez-Gonzáleza, A.; Bermúdez de Castro, J.M.; Carbonell, E.; Arsuagac, J.L. (2013). "Reassessing the age of Atapuerca-TD6 (Spain): new paleomagnetic results" (PDF). Journal of Archaeological Science. 40 (12): 4586–4595. Bibcode:2013JArSc..40.4586P. doi:10.1016/j.jas.2013.06.013.
- Duval, Mathieu; Grün, Rainer; Parés, Josep M.; Martín-Francés, Laura; Campaña, Isidoro; Rosell, Jordi; Shao, Qingfeng; Arsuaga, Juan Luis; Carbonell, Eudald; Bermúdez de Castro, José María (2018). "The first direct ESR dating of a hominin tooth from Atapuerca Gran Dolina TD-6 (Spain) supports the antiquity of Homo antecessor". Quaternary Geochronology. 47: 120–137. Bibcode:2018QuGeo..47..120D. doi:10.1016/j.quageo.2018.05.001.
- Duval, M.; Arnold, L. J.; Demuro, M.; Parés, J. M.; Campaña, I.; Carbonell, E.; de Castro, J. M. B. (2022). "New chronological constraints for the lowermost stratigraphic unit of Atapuerca Gran Dolina (Burgos, N Spain)". Quaternary Geochronology. 71: 101292. Bibcode:2022QuGeo..7101292D. doi:10.1016/j.quageo.2022.101292. S2CID 248162093.
- Toro-Moyano, I.; Martínez-Navarro, B.; Agustí, J.; Souday, C.; Bermúdez; de Castro, J.M.; Martinón-Torres, M.; Fajardo, B.; Duval, M.; Falguères, C.; Oms, O.; Parés, J.M.; Anadón, P.; Julià, R.; García-Aguilar, J.M.; Moigne, A.M.; Espigares, M.P.; Ros-Montoya, S.; Palmqvist, P. (2013). "The oldest human fossil in Europe, from Orce (Spain)". Journal of Human Evolution. 65 (1): 1–9. doi:10.1016/j.jhevol.2013.01.012. hdl:10261/84112. PMID 23481345.
- Moyano, I. T.; Barsky, D. (2011). "The archaic stone tool industry from Barranco León and Fuente Nueva 3, (Orce, Spain): Evidence of the earliest hominin presence in southern Europe". Quaternary International. 243 (1): 80–91. Bibcode:2011QuInt.243...80M. doi:10.1016/j.quaint.2010.12.011.
- Stringer, C. (2012). "What makes a modern human". Nature. 485 (7396): 33–35. Bibcode:2012Natur.485...33S. doi:10.1038/485033a. PMID 22552077. S2CID 4420496.
- Arambourg, C. (1955). "Le gisement de Ternifine et l'Atlanthropus" [The deposits of Ternifine and Atlantanthropus]. Bulletin de la Société préhistorique française (in French). 52 (52): 94–95. doi:10.3406/bspf.1955.3159. JSTOR 27914994.
- Antón, S. C.; Middleton, E. R. (2014). "Homo ergaster". Encyclopedia of Global Archaeology. Springer. p. 3462. doi:10.1007/978-1-4419-0465-2_688. ISBN 978-1-4419-0426-3.
- Hublin, J.-J. (2001). "Northwestern African Middle Pleistocene hominids and their bearing on the emergence of Homo sapiens". In Barham, L.; Robson-Brown, K. (eds.). Human Roots: Africa and Asia in the Middle Pleistocene (PDF). Western Academic and Specialist Press. pp. 116–118. ISBN 978-0-9535418-4-3.
- de Castro, J.-M. B.; María-Torres, M.; Gómez-Robles, A.; Prado, L.; Sarmiento, S. (2007). "Comparative analysis of the Gran Dolina-TD6 (Spain) and Tighennif (Algeria) hominin mandibles". Bulletins et Mémoires de la Société of Anthropologie de Paris. 19 (3–4): 149–167. doi:10.4000/bmsap.4623.
- Sarmiento, E. E.; Mowbray, K.; Sawyer, G. J.; Milner, R.; Deak, V.; Tattersall, I. (2007). "Homo antecessor". The Last Human: A Guide to Twenty-two Species of Extinct Humans. Yale University Press. pp. 190–191. ISBN 978-0-300-10047-1.
- Klein, R. (2009). "Hominin dispersals in the Old World". In Scarre, C. (ed.). The Human Past (2nd ed.). Thames and Hudson. p. 108. ISBN 978-0-500-29063-7.
- Freidline, S. E.; Gunz, P.; Harvati, K.; Hublin, J.-J. (2013). "Evaluating developmental shape changes in Homo antecessor subadult facial morphology". Journal of Human Evolution. 65 (4): 404–423. doi:10.1016/j.jhevol.2013.07.012. PMID 23998458.
- de Castro, J. M. B.; Martinón‐Torres, M.; Arsuaga, J. L.; Carbonell, E. (2017). "Twentieth anniversary of Homo antecessor (1997‐2017): a review". Evolutionary Anthropology. 26 (4): 157–171. doi:10.1002/evan.21540. PMID 28815959. S2CID 11442202.
- Welker, Frido; Ramos-Madrigal, Jazmín; Gutenbrunner, Petra; Mackie, Meaghan; Tiwary, Shivani; Rakownikow Jersie-Christensen, Rosa; Chiva, Cristina; Dickinson, Marc R.; Kuhlwilm, Martin; de Manuel, Marc; Gelabert, Pere; Martinón-Torres, María; Margvelashvili, Ann; Arsuaga, Juan Luis; Carbonell, Eudald; Marques-Bonet, Tomas; Penkman, Kirsty; Sabidó, Eduard; Cox, Jürgen; Olsen, Jesper V.; Lordkipanidze, David; Racimo, Fernando; Lalueza-Fox, Carles; Bermúdez de Castro, José María; Willerslev, Eske; Cappellini, Enrico (2020). "The dental proteome of Homo antecessor". Nature. 580 (7802): 235–238. Bibcode:2020Natur.580..235W. doi:10.1038/s41586-020-2153-8. ISSN 1476-4687. PMC 7582224. PMID 32269345. S2CID 214736611.
- García-Campos, C.; Martinén-Torres, M.; Modesto-Mata, M.; Martín-Francés, L.; de Pinillos, M. M.; de Castro, J. M. B. (2021). "Indicators of sexual dimorphism in Homo antecessor permanent canines". Journal of Anthropological Sciences. 99 (99): 1–18. doi:10.4436/JASS.99001. PMID 33707343. S2CID 232206781.
- Allen, J. S.; Damasio, H.; Grabowski, T. J. (2002). "Normal neuroanatomical variation in the human brain: an MRI-volumetric study". American Journal of Physical Anthropology. 118 (4): 351. doi:10.1002/ajpa.10092. PMID 12124914. S2CID 21705705.
- Pablos, A.; Lorenzo; Martínez, C.; de Castro, J. M. B.; Martinón-Torres, M.; Carbonell, E.; Arsuaga, J. L. (2012). "New foot remains from the Gran Dolina-TD6 Early Pleistocene site (Sierra de Atapuerca, Burgos, Spain)". Journal of Human Evolution. 63 (4): 610–623. doi:10.1016/j.jhevol.2012.06.008. PMID 22921478.
- García-Martínez, Daniel; Green, David J.; Bermúdez de Castro, José María (2021). "Evolutionary development of the Homo antecessor scapulae (Gran Dolina site, Atapuerca) suggests a modern-like development for Lower Pleistocene Homo". Scientific Reports. 11 (4102): 4102. Bibcode:2021NatSR..11.4102G. doi:10.1038/s41598-021-83039-w. PMC 7892855. PMID 33602966.
- de Castro, J.-M. B.; Martinón-Torres, M.; Prado, L.; Gómez-Robles, A.; Rosell, J.; López-Polín, L.; Arsuaga, J. L.; Carbonell, E. (2010). "New immature hominin fossil from European Lower Pleistocene shows the earliest evidence of a modern human dental development pattern". Proceedings of the National Academy of Sciences. 107 (26): 11739–11744. Bibcode:2010PNAS..10711739B. doi:10.1073/pnas.1006772107. PMC 2900696. PMID 20547843.
- Martín-Francés, L.; Martinón-Torres, M.; de Pinillos, M. M.; Bayle, P.; Fernández-Colón, P.; García-Campos, C.; Modesto-Mata, M.; Carbonell, E.; Arsuaga, J. L.; de Castro, J. M. B. (2020). "Ectopic maxillary third molar in Early Pleistocene Homo antecessor from Atapuerca-Gran Dolina site (Burgos, Spain)". American Journal of Biological Anthropology. 171 (4): 733–741. doi:10.1002/ajpa.24010. PMID 31943140. S2CID 210333472.
- López-Valverde, A.; López-Cristiá, M.; Gómez de Diego, R. (2012). "Europe's oldest jaw: evidence of oral pathology". British Dental Journal. 212 (5): 243–245. doi:10.1038/sj.bdj.2012.176. PMID 22402544. S2CID 22346193.
- Martín-Francés, L.; Martinón-Torres, M.; Gracia-Téllez, A.; de Castro, J. M. B. (2016). "Evidence of trauma in a ca. 1-million-year-old patella of Homo antecessor, Gran Dolina-Atapuerca (Spain)" (PDF). Comptes Rendus Palevol. 15 (8): 1011–1016. Bibcode:2016CRPal..15.1011M. doi:10.1016/j.crpv.2016.04.014.
- Martin-Francés, L.; Martinon-Torres, M.; Gracia-Téllez, A.; de Castro, J. M. B (2015). "Evidence of stress fracture in a Homo antecessor metatarsal from Gran Dolina site (Atapuerca, Spain)". International Journal of Osteoarchaeology. 25 (4): 564–573. doi:10.1002/oa.2310.
- Moncel, M.-H.; Santagata, C.; Pereira, A.; Nomade, S.; Voinchet, P.; Bahain, J.-J.; Daujeard, C.; Curci, A.; Lemorini, C.; Hardy, B.; Eramo, G.; Berto, C.; Raynal, J.-P.; Arzarello, M.; Mecozzi, B.; Iannucci, A.; Sardella, R.; Allegretta, I.; Delluniversità, E.; Terzano, R.; Dugas, P.; Jouanic, G.; Queffelec, A.; d'Andrea, A.; Valentini, R.; Minucci, E.; Carpentiero, L.; Piperno, M. (2020). "The origin of early Acheulean expansion in Europe 700 ka ago: new findings at Notarchirico (Italy)". Scientific Reports. 10 (1): 13802. Bibcode:2020NatSR..1013802M. doi:10.1038/s41598-020-68617-8. PMC 7429832. PMID 32796860.
- Roebroeks, W.; Villa, P. (2011). "On the earliest evidence for habitual use of fire in Europe". Proceedings of the National Academy of Sciences. 108 (13): 5209–5214. Bibcode:2011PNAS..108.5209R. doi:10.1073/pnas.1018116108. PMC 3069174. PMID 21402905.
- Rhodes, S.E.; Walker, M.J.; López-Jiménez, A.; López-Martínez, M.; Haber-Uriarte, M.; Fernández-Jalvo, Y.; Chazan, M. (2016). "Fire in the Early Palaeolithic: Evidence from burnt small mammal bones at Cueva Negra del Estrecho del Río Quípar, Murcia, Spain". Journal of Archaeological Science: Reports. 9: 427–436. Bibcode:2016JArSR...9..427R. doi:10.1016/j.jasrep.2016.08.006.
- Saladié, P.; Huguet, R.; Díez, C.; Rodríguez-Hidalgo, A.; Cáceres, I.; Vallverdú, J.; Rosell, J.; de Castro, J. M. B.; Carbonell, E. (2011). "Carcass transport decisions in Homo antecessor subsistence strategies". Journal of Human Evolution. 61 (4): 425–446. doi:10.1016/j.jhevol.2011.05.012. PMID 21802117. S2CID 36583909.
- Blasco, R.; Blain, H.-A.; Rosell, J.; Díez, J. C.; Huguet, R.; Rodríguez, J.; Arsuaga, J. L.; de Castro, J. M. B.; Carbonell, E. (2011). "Earliest evidence for human consumption of tortoises in the European Early Pleistocene from Sima del Elefante, Sierra de Atapuerca, Spain". Journal of Human Evolution. 61 (4): 503–509. doi:10.1016/j.jhevol.2011.06.002. PMID 21807397.
- Allué, E.; Cáceres, I.; Expósito, I.; Canals, A.; Rodríguez, A.; Rosell, J.; de Castro, J. M. B.; Carbonell, E. (2015). "Celtis remains from the Lower Pleistocene of Gran Dolina, Atapuerca (Burgos, Spain)". Journal of Archaeological Science. 53: 570–577. Bibcode:2015JArSc..53..570A. doi:10.1016/j.jas.2014.11.016.
- Pérez-Pérez, A.; Lozano, M.; Romero, A.; Martínez, L. M.; Galbany, J.; Pinilla, B.; Estebaranz-Sánchez, F.; de Castro, J. M. B.; Carbonell, E.; Arsuaga, J. L. (2017). "The diet of the first Europeans from Atapuerca". Scientific Reports. 7: 43319. Bibcode:2017NatSR...743319P. doi:10.1038/srep43319. PMC 5327419. PMID 28240290.
- Carbonell, E.; Cáceres, I.; Lozano, M.; Saladié, P.; Rosell, J.; Lorenzo, C.; Huguet, R.; Canals, A.; de Castro, J.-M. B. (2010). "Cultural cannibalism as a paleoeconomic system in the European Lower Pleistocene". Current Anthropology. 51 (4): 539–549. doi:10.1086/653807. S2CID 1311044.
- Rodríguez, J.; Guillermo, Z.-R.; Ana, M. (2019). "Does optimal foraging theory explain the behavior of the oldest human cannibals?". Journal of Human Evolution. 131: 228–239. doi:10.1016/j.jhevol.2019.03.010. PMID 31182203. S2CID 149855232.
Further reading
- de Castro, J.-M. B. (2002). El chico de la Gran Dolina [The Gran Dolina boy] (in Spanish). Crítica. ISBN 978-84-8432-317-4.