HCTU
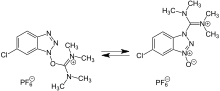
HCTU is an aminium coupling reagent used in peptide synthesis. It is analogous to HBTU.[1] The HOBt moiety has a chlorine in the 6 position which improves reaction rates and the synthesis of difficult couplings[2][3]
 | |
| Names | |
|---|---|
| IUPAC name
O-(1H-6-Chlorobenzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate | |
| Other names
2-(6-Chloro-1H-benzotriazole-1-yl)-1,1,3,3-tetramethylaminium hexafluorophosphate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.116.975 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C11H15ClF6N5OP | |
| Molar mass | 413.69 g·mol−1 |
| Appearance | White to off white powder |
| Melting point | >185 °C |
| Hazards | |
| GHS labelling: | |
  | |
| Warning | |
| H228, H302, H315, H319, H335 | |
| P210, P240, P241, P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P370+P378, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- Marder, Oleg; Shvo, Youval; Albericio, Fernando (2003-08-12). "HCTU and TCTU: New Coupling Reagents — Development and Industrial Aspects". ChemInform. 34 (32). doi:10.1002/chin.200332258. ISSN 1522-2667.
- Hood, Christina A.; Fuentes, German; Patel, Hirendra; Page, Karen; Menakuru, Mahendra; Park, Jae H. (2008-01-01). "Fast conventional Fmoc solid-phase peptide synthesis with HCTU". Journal of Peptide Science. 14 (1): 97–101. CiteSeerX 10.1.1.595.917. doi:10.1002/psc.921. ISSN 1099-1387. PMID 17890639. S2CID 24163278.
- Sabatino, Giuseppina; Mulinacci, Barbara; Alcaro, Maria C.; Chelli, Mario; Rovero, Paolo; Papini, Anna M. (2002-03-01). "Assessment of new 6-Cl-HOBt based coupling reagents for peptide synthesis. Part 1: Coupling efficiency study". Letters in Peptide Science. 9 (2–3): 119–123. doi:10.1007/bf02576873. ISSN 0929-5666. S2CID 29545816.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.