SLBP
Histone RNA hairpin-binding protein or stem-loop binding protein (SLBP) is a protein that in humans is encoded by the SLBP gene.[5][6][7]
| SLBP | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | SLBP, HBP, stem-loop binding protein | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 602422 MGI: 108402 HomoloGene: 31389 GeneCards: SLBP | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Species distribution
SLBP has been cloned from humans, C. elegans, D. melanogaster, X. laevis, and sea urchins. The full length human protein has 270 amino acids (31 kDa) with a centrally located RNA binding domain (RBD). The 75 amino acid RBD is well conserved across species, however the remainder of SLBP is highly divergent in most organisms and not homologous to any other protein in the eukaryotic genomes.
Function
This gene encodes a protein that binds to the histone 3' UTR stem-loop structure in replication-dependent histone mRNAs. Histone mRNAs do not contain introns or polyadenylation signals, and are processed by a single endonucleolytic cleavage event downstream of the stem-loop. The stem-loop structure is essential for efficient processing of the histone pre-mRNA but this structure also controls the transport, translation and stability of histone mRNAs. SLBP expression is regulated during S-phase of the cell cycle, increasing more than 10-fold during the latter part of G1.
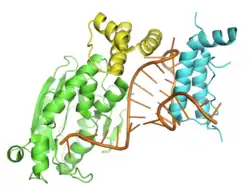
All SLBP proteins are capable of forming a highly stable complex with histone stem-loop RNA. Complex formation with the histone mRNA stem-loop is achieved by a novel three-helix bundle fold. SLBP proteins also recognize the tetraloop structure of the histone hairpin, the base of the stem, and the 5' flanking region. The crystal structure of human SLBP in complex with the stem-loop RNA as well as the exonuclease Eri1 reveals that the Arg181 residue of SLBP specifically interacts with the second guanine base in the RNA stem.[8] The rest of the protein is intrinsically disordered in fruit-flies as well as in humans. A unique feature of the SLBP RBD is that it is phosphorylated in its RNA binding domain at the Thr171 residue. The SLBP RBD also undergoes proline isomerization about this sequence and is a substrate for the prolyl isomerase Pin1. The N-terminal domain of human SLBP is required for translation activation of histone mRNAs via its interaction with SLIP1. SLBP also interacts with the CBP80 associated protein CTIF to facilitate rapid degradation of histone mRNAs. SLBP is a phosphoprotein and besides T171, it is also phosphorylated at Ser7, Ser20, Ser23, Thr60, Thr61 in mammalian cells. The phosphorylation at Thr60 is mediated by CK2 and Thr61 is by Cyclin A/Cdk1.[7]
References
- GRCh38: Ensembl release 89: ENSG00000163950 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000004642 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Martin F, Schaller A, Eglite S, Schumperli D, Muller B (Mar 1997). "The gene for histone RNA hairpin binding protein is located on human chromosome 4 and encodes a novel type of RNA binding protein". EMBO J. 16 (4): 769–78. doi:10.1093/emboj/16.4.769. PMC 1169678. PMID 9049306.
- McCombie WR, Martin-Gallardo A, Gocayne JD, FitzGerald M, Dubnick M, Kelley JM, Castilla L, Liu LI, Wallace S, Trapp S (August 1992). "Expressed genes, Alu repeats and polymorphisms in cosmids sequenced from chromosome 4p16.3". Nature Genetics. 1 (5): 348–53. doi:10.1038/ng0892-348. PMID 1338771. S2CID 6716635.
- "Entrez Gene: SLBP stem-loop (histone) binding protein".
- Dazhi Tan; William F. Marzluff; Zbigniew Dominski; Liang Tong (Jan 2013). "Structure of Histone mRNA Stem-Loop, Human Stem-Loop Binding Protein, and 3′hExo Ternary Complex". Science. 339 (6117): 318–321. doi:10.1126/science.1228705. PMC 3552377. PMID 23329046.
Further reading
- Choe J, Mi Kim K, Park S, Kyung Lee Y, Song O-K, Kim MK, Lee BG, Song HK, Kim YK (2013). "Rapid degradation of replication-dependent histone mRNAs largely occurs on mRNAs bound by nuclear cap-binding proteins 80 and 20". Nucleic Acids Research. 41 (2): 1307–1318. doi:10.1093/nar/gks1196. PMC 3553978. PMID 23234701.
- Bansal N, Zhang M, Bhaskar A, Itotia P, Lee E, Shlyakhtenko LS, Lam TT, Fritz A, Berezney R, Lyubchenko YL, Stafford WF, Thapar R (January 2013). "Assembly of the SLIP1-SLBP complex on histone mRNA requires heterodimerization and sequential binding of SLBP followed by SLIP1". Biochemistry. 52 (3): 520–536. doi:10.1021/bi301074r. PMC 3580866. PMID 23286197.
- Martin L, Meier M, Lyons SM, Sit RV, Marzluff WF, Quake SR, Chang HY (December 2012). "Systematic reconstruction of RNA functional motifs with high-throughput microfluidics". Nature Methods. 9 (12): 1192–4. doi:10.1038/nmeth.2225. PMC 3863600. PMID 23142872.
- Krishnan N, Lam TT, Fritz A, Rempinski D, O'Loughlin K, Minderman H, Berezney R, Marzluff WF, Thapar R (November 2012). "The Prolyl Isomerase Pin1 Targets Stem-Loop Binding Protein (SLBP) To Dissociate the SLBP-Histone mRNA Complex Linking Histone mRNA Decay with SLBP Ubiquitination". Mol. Cell. Biol. 32 (21): 4306–22. doi:10.1128/MCB.00382-12. PMC 3486140. PMID 22907757.
- Zhang M, Lam TT, Tonelli M, Marzluff WF, Thapar R (April 2012). "Interaction of the histone mRNA hairpin with stem-loop binding protein (SLBP) and regulation of the SLBP-RNA complex by phosphorylation and proline isomerization". Biochemistry. 51 (15): 3215–31. doi:10.1021/bi2018255. PMC 3328597. PMID 22439849.
- Borchers CH, Thapar R, Petrotchenko EV, Torres MP, Speir JP, Easterling M, Dominski Z, Marzluff WF (February 2006). "Combined top-down and bottom-up proteomics identifies a phosphorylation site in stem-loop-binding proteins that contributes to high-affinity RNA binding". Proc. Natl. Acad. Sci. U.S.A. 103 (9): 3094–9. Bibcode:2006PNAS..103.3094B. doi:10.1073/pnas.0511289103. PMC 1413926. PMID 16492733.
- Thapar R, Marzluff WF, Redinbo MR (July 2004). "Electrostatic contribution of serine phosphorylation to the Drosophila SLBP--histone mRNA complex". Biochemistry. 43 (29): 9401–12. doi:10.1021/bi036315j. PMID 15260483.
- Thapar R, Mueller GA, Marzluff WF (July 2004). "The N-terminal domain of the Drosophila histone mRNA binding protein, SLBP, is intrinsically disordered with nascent helical structure". Biochemistry. 43 (29): 9390–400. doi:10.1021/bi036314r. PMID 15260482.
- Wang ZF, Whitfield ML, Ingledue TC, Dominski Z, Marzluff WF (December 1996). "The protein that binds the 3' end of histone mRNA: a novel RNA-binding protein required for histone pre-mRNA processing". Genes Dev. 10 (23): 3028–40. doi:10.1101/gad.10.23.3028. PMID 8957003.
- Dominski Z, Zheng LX, Sanchez R, Marzluff WF (May 1999). "Stem-loop binding protein facilitates 3'-end formation by stabilizing U7 snRNP binding to histone pre-mRNA". Mol. Cell. Biol. 19 (5): 3561–70. doi:10.1128/mcb.19.5.3561. PMC 84148. PMID 10207079.
- Whitfield ML, Zheng LX, Baldwin A, Ohta T, Hurt MM, Marzluff WF (June 2000). "Stem-loop binding protein, the protein that binds the 3' end of histone mRNA, is cell cycle regulated by both translational and posttranslational mechanisms". Mol. Cell. Biol. 20 (12): 4188–98. doi:10.1128/MCB.20.12.4188-4198.2000. PMC 85788. PMID 10825184.
- Dominski Z, Erkmann JA, Greenland JA, Marzluff WF (March 2001). "Mutations in the RNA binding domain of stem-loop binding protein define separable requirements for RNA binding and for histone pre-mRNA processing". Mol. Cell. Biol. 21 (6): 2008–17. doi:10.1128/MCB.21.6.2008-2017.2001. PMC 86798. PMID 11238936.
- Allard P, Champigny MJ, Skoggard S, Erkmann JA, Whitfield ML, Marzluff WF, Clarke HJ (December 2002). "Stem-loop binding protein accumulates during oocyte maturation and is not cell-cycle-regulated in the early mouse embryo". J. Cell Sci. 115 (Pt 23): 4577–86. doi:10.1242/jcs.00132. PMC 5115915. PMID 12415002.
- Zheng L, Dominski Z, Yang XC, Elms P, Raska CS, Borchers CH, Marzluff WF (March 2003). "Phosphorylation of stem-loop binding protein (SLBP) on two threonines triggers degradation of SLBP, the sole cell cycle-regulated factor required for regulation of histone mRNA processing, at the end of S phase". Mol. Cell. Biol. 23 (5): 1590–601. doi:10.1128/MCB.23.5.1590-1601.2003. PMC 151715. PMID 12588979.
- Koseoglu MM, Graves LM, Marzluff WF (July 2008). "Phosphorylation of threonine 61 by cyclin a/Cdk1 triggers degradation of stem-loop binding protein at the end of S phase". Mol. Cell. Biol. 28 (14): 4469–79. doi:10.1128/MCB.01416-07. PMC 2447125. PMID 18490441.
- Sànchez R, Marzluff WF (October 2002). "The stem-loop binding protein is required for efficient translation of histone mRNA in vivo and in vitro". Mol. Cell. Biol. 22 (20): 7093–104. doi:10.1128/mcb.22.20.7093-7104.2002. PMC 139811. PMID 12242288.
- Zhao X, McKillop-Smith S, Müller B (December 2004). "The human histone gene expression regulator HBP/SLBP is required for histone and DNA synthesis, cell cycle progression and cell proliferation in mitotic cells". J. Cell Sci. 117 (Pt 25): 6043–51. doi:10.1242/jcs.01523. PMID 15546920.
- Erkmann JA, Wagner EJ, Dong J, Zhang Y, Kutay U, Marzluff WF (June 2005). "Nuclear import of the stem-loop binding protein and localization during the cell cycle". Mol. Biol. Cell. 16 (6): 2960–71. doi:10.1091/mbc.E04-11-1023. PMC 1142439. PMID 15829567.