Heated bath
A heated bath is used in the laboratory to allow a chemical reaction to occur at an elevated temperature.[1]
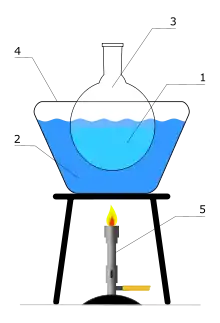
In contrast to traditional Bunsen burners, heated baths use liquids to transfer heat to the reaction vessel. This is achieved using a high-boiling point liquid inside a thermally conducting bath (usually made of metal). Water and silicone oil are the most commonly used fluids. A water bath is used for temperatures up to 100 °C. An oil bath is employed for temperatures over up to and above 100 °C.
The heated bath is heated on an electric hot plate, or with a Bunsen burner. The reaction vessel (Florence flask, Erlenmeyer flask, or beaker) is immersed in the heated bath. A thermometer is usually kept in the fluid to monitor the temperature.
See also
- Bain-marie, a.k.a. double boiler
- Heat bath
- Sand bath
References
- Furniss, Brian S.; Vogel, Arthur Israel, eds. (2009). Vogel's textbook of practical organic chemistry (New. ed., 5. ed., rev. [Nachdr.] ed.). Harlow: Pearson/Prentice Hall. ISBN 978-0-582-46236-6.