Heptalene
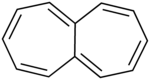
Heptalene is a polycyclic hydrocarbon with chemical formula C12H10, composed of two fused cycloheptatriene rings. It is an unstable, non-planar compound which is non-aromatic.[1][2] The dianion, however, satisfies Hückel's rule, is thermally stable, and is planar.[3]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Heptalene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H10 | |
| Molar mass | 154.212 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
See also
References
- Gottarelli, Giovanni; Hansen, Hans-Jürgen; Spada, Gian Piero; Weber, Roland H. (1987). "A Liquid-Crystal Study of Heptalene". Helvetica Chimica Acta. 70 (2): 430. doi:10.1002/hlca.19870700222.
- Boyd, G.V. (1966). "The aromaticity of pentalene, heptalene and related bicyclic hydrocarbons". Tetrahedron. 22 (10): 3409. doi:10.1016/S0040-4020(01)92529-3.
- Oth, Jean F. M.; Müllen, Klaus; Königshofen, Heinrich; Wassen, Jürgen; Vogel, Emanuel (1974). "The Dianion of Heptalene". Helvetica Chimica Acta. 57 (8): 2387. doi:10.1002/hlca.19740570811.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.