Hexafluoroisobutylene
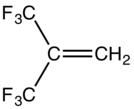
Hexafluoroisobutylene is an organofluorine compound with the formula (CF3)2C=CH2. This colorless gas is structurally similar to isobutylene. It is used as a comonomer in the production of modified polyvinylidene fluoride. It is produced in a multistep process starting with the reaction of acetic anhydride with hexafluoroacetone.[1] It is oxidized by sodium hypochlorite to hexafluoroisobutylene oxide. As expected, it is a potent dienophile.[2]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
3,3,3-Trifluoro-2-(trifluoromethyl)prop-1-ene | |
| Other names
hexafluoroisobutene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.006.219 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H2F6 | |
| Molar mass | 164.050 g·mol−1 |
| Appearance | colorless gas |
| Boiling point | 14.1 °C (57.4 °F; 287.2 K) |
| Hazards | |
| GHS labelling: | |
   | |
| Danger | |
| H280, H331, H341, H372 | |
| P201, P202, P260, P261, P264, P270, P271, P281, P304+P340, P308+P313, P311, P314, P321, P403+P233, P405, P410+P403, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
See also
References
- Günter Siegemund; Werner Schwertfeger; Andrew Feiring; Bruce Smart; Fred Behr; Herward Vogel; Blaine McKusick; Peer Kirsch (2016). "Fluorine Compounds, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. pp. 1–56. doi:10.1002/14356007.a11_349.pub2. ISBN 978-3-527-30673-2.
- Murphy, Peter M. (2013). "The chemistry and utility of hexafluoroisobutylene (HFIB) and hexafluoroisobutylene oxide (HFIBO)". Journal of Fluorine Chemistry. 156: 345–362. doi:10.1016/j.jfluchem.2013.07.015.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.