Hexasulfur
Hexasulfur is an inorganic chemical with the chemical formula S6.
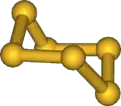 | |
| Names | |
|---|---|
Systematic IUPAC name
| |
| Other names
Hexasulfur | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| S6 | |
| Molar mass | 192.36 g·mol−1 |
| Appearance | Vivid, orange, opaque crystals |
| Related compounds | |
Related compounds |
Octasulfur |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Nomenclature
The name hexasulfur is the most commonly used and preferred IUPAC name and is constructed according to the compositional nomenclature, and cyclohexasulfane. It is also the final member of the thiane heterocyclic series, where every carbon atom is substituted with a sulfur atom, thus the systematic name hexathiane, a valid IUPAC name, is constructed according to the substitutive nomenclature. Another valid IUPAC systematic name cyclo-hexasulfur is constructed according to the additive nomenclature.
Structure
This chemical consists of rings of 6 sulfur atoms. It is thus a simple cyclosulfane and an allotrope of sulfur. Hexasulfur adopts a chair configuration similar to that of cyclohexane, with bond angles of 102.2°. The sulfur atoms are equivalent.[2]
References
- "S6 - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 656. ISBN 978-0-08-037941-8.