Hinsberg reaction
The Hinsberg reaction is a chemical test for the detection of primary, secondary and tertiary amines. The reaction was first described by Oscar Hinsberg in 1890.[1][2] In this test, the amine is shaken well with the Hinsberg reagent (benzenesulfonyl chloride) in the presence of aqueous alkali (either KOH or NaOH). A primary amine will form a soluble sulfonamide salt. Acidification of this salt then precipitates the sulfonamide of the primary amine. A secondary amine in the same reaction will directly form an insoluble sulfonamide. A tertiary amine will not react with the original reagent (benzene sulfonyl chloride) and will remain insoluble. After adding dilute acid this insoluble amine is converted to a soluble ammonium salt. In this way the reaction can distinguish between the three types of amines.[3]
Tertiary amines are able to react with benzenesulfonyl chloride under a variety of conditions; the test described above is not absolute. The Hinsberg test for amines is valid only when reaction speed, concentration, temperature, and solubility are taken into account.[4]
Reactions
Amines serve as nucleophiles in attacking the sulfonyl chloride electrophile, displacing chloride. The sulfonamides resulting from primary and secondary amines are poorly soluble and precipitate as solids from solution.
- PhSO2Cl + 2 RR'NH → PhSO2NRR' + [RR'NH2+]Cl−
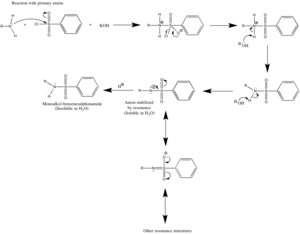
For primary amines (R' = H), the initially formed sulfonamide is deprotonated by base to give a water-soluble sulfonamide salt (Na[PhSO2NR]).
- PhSO2N(H)R + NaOH → Na+[PhSO2NR−] + H2O
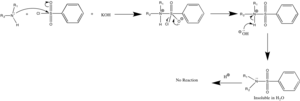
Tertiary amines promote hydrolysis of the sulfonyl chloride functional group, which affords water-soluble sulfonate salts.
- PhSO2Cl + R3N + H2O → R3NH+[PhSO−
3] + HCl

References
- O. Hinsberg: Ueber die Bildung von Säureestern und Säureamiden bei Gegenwart von Wasser und Alkali, in: Ber. Dtsch. Chem. Ges. 1890, 23, 2962–2965; doi:10.1002/cber.189002302215
- O. Hinsberg, J. Kessler: Ueber die Trennung der primären und secundären Aminbasen, in: Ber. Dtsch. Chem. Ges. 1905, 38, 906–911; doi:10.1002/cber.190503801161
- "The systematic identification of organic compounds" 4th ed. by Ralph L. Shriner, Reynold C. Fuson, and David Y. Curtin. John Wiley & Sons, Inc., New York, 1956. doi:10.1002/jps.3030450636 and more recent editions.
- Gambill, C. R. (1972). "Benzenesulfonyl chloride does react with tertiary amines. The Hinsberg test in proper prospective". Journal of Chemical Education. 49 (4): 287. Bibcode:1972JChEd..49..287G. doi:10.1021/ed049p287.
External links
- Laboratory procedure: science.csustan.edu