Homocitric acid
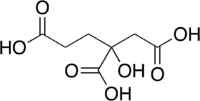
Homocitric acid is an organic compound with the formula HOC(CO2H)(CH2CO2H)(C2H4CO2H). This tricarboxylic acid occurs naturally as a component of the iron-molybdenum cofactor of certain nitrogenase proteins.[1] Biochemists often refer to this cofactor as homocitrate, which is the conjugate bases that predominate in neutral aqueous solutions of this species.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Hydroxybutane-1,2,4-tricarboxylic acid | |
| Other names
Homocitric acid Homocitrate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C7H10O7 | |
| Molar mass | 206.150 g·mol−1 |
| Appearance | Colorless solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
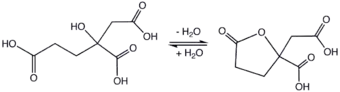
The molecule is related to citric acid by the addition of one methylene unit, hence the prefix "homo." Unlike citric acid, homocitric acid is chiral. The acid exists in equilibrium with the lactone.
See also
References
- Rees, Douglas C. (2002). "Great Metalloclusters in Enzymology" (PDF). Annual Review of Biochemistry. 71: 221–46. doi:10.1146/annurev.biochem.71.110601.135406. PMID 12045096.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.