Homophthalic acid
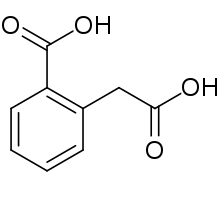
Homophthalic acid is a dicarboxylic acid with the formula C6H4(CO2H)CH2CO2H. It is a colorless solid. The compounds can be prepared by the Willgerodt reaction from 2-acetylbenzoic acid.[3]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-(Carboxymethyl)benzoic acid | |
| Other names
α-Carboxy-o-toluic acid Carboxyphenyl acetic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.740 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C9H8O4 | |
| Molar mass | 180.159 g·mol−1 |
| Appearance | Off-white to light yellow or pale green |
| Melting point | 181 °C (358 °F; 454 K)[2] |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
One of the uses is in the preparation of the NSAID tesicam.
References
- http://ops.rsc.org/Compounds/Get/670357%5B%5D
- Homophthalic Acid | C9H8O4 | Chemspider
- Schwenk, Erwin; Papa, Domenick (1946). "Preparation of Aryl Aliphatic Acids by the Modified Willgerodt Reaction". The Journal of Organic Chemistry. 11 (6): 798–802. doi:10.1021/jo01176a023. PMID 20282506.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.