Homoplasy
Homoplasy, in biology and phylogenetics, is the term used to describe a feature that has been gained or lost independently in separate lineages over the course of evolution. This is different from homology, which is the term used to characterize the similarity of features that can be parsimoniously explained by common ancestry.[1] Homoplasy can arise from both similar selection pressures acting on adapting species, and the effects of genetic drift.[2][3]
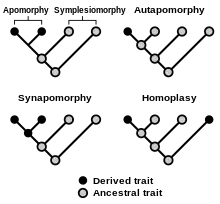
Most often, homoplasy is viewed as a similarity in morphological characters. However, homoplasy may also appear in other character types, such as similarity in the genetic sequence,[4][5] life cycle types[6] or even behavioral traits.[7][5]
Etymology
The term homoplasy was first used by Ray Lankester in 1870.[8] The corresponding adjective is either homoplasic or homoplastic. It is derived from the two Ancient Greek words ὁμός (homós), meaning "similar, alike, the same", and πλάσσω (plássō), meaning "to shape, to mold".[9][10][11][4]
Parallelism and convergence
Parallel and convergent evolution lead to homoplasy when different species independently evolve or gain apparently identical features, which are different from the feature inferred to have been present in their common ancestor. When the similar features are caused by an equivalent developmental mechanism, the process is referred to as parallel evolution.[12][13] The process is called convergent evolution when the similarity arises from different developmental mechanisms.[13][14] These types of homoplasy may occur when different lineages live in comparable ecological niches that require similar adaptations for an increase in fitness. An interesting example is that of the marsupial moles (Notoryctidae), golden moles (Chrysochloridae) and northern moles (Talpidae). These are mammals from different geographical regions and lineages, and have all independently evolved very similar burrowing characteristics (such as cone-shaped heads and flat frontal claws) to live in a subterranean ecological niche.[15]
Reversion
In contrast, reversal (a.k.a. vestigialization) leads to homoplasy through the disappearance of previously gained features.[16] This process may result from changes in the environment in which certain gained characteristics are no longer relevant, or have even become costly.[17][3] This can be observed in subterranean and cave-dwelling animals by their loss of sight,[15][18] in cave-dwelling animals through their loss of pigmentation,[18] and in both snakes and legless lizards through their loss of limbs.[19][20]
Distinguishing homology from homoplasy
Homoplasy, especially the type that occurs in more closely related phylogenetic groups, can make phylogenetic analysis more challenging. Phylogenetic trees are often selected by means of parsimony analysis.[21][22] These analyses can be done with phenotypic characters, as well as DNA sequences.[23] Using parsimony analysis, the hypothesis of relationships that requires the fewest (or least costly) character state transformations is preferred over alternative hypotheses. Evaluation of these trees may become a challenge when clouded by the occurrence of homoplasy in the characters used for the analysis. The most important approach to overcoming these challenges is to increase the number of independent (non-pleiotropic, non-linked) characteristics used in the phylogenetic analysis. Along with parsimony analysis, one could perform a likelihood analysis, where the most likely tree, given a particular model of evolution, is selected, and branch lengths are inferred.
According to the cladistic interpretation, homoplasy is invoked when the distribution of a character state cannot be explained parsimoniously (without extra inferred character state transformations between the terminals and their ancestral node) on a preferred phylogenetic hypothesis - that is, the feature in question arises (or disappears) at more than one point on the tree.[16]
In the case of DNA sequences, homoplasy is very common due to the redundancy of the genetic code. An observed homoplasy may simply be the result of random nucleotide substitutions accumulating over time, and thus may not need an adaptationist evolutionary explanation.[5]
Examples and applications of homoplasy
There are numerous documented examples of homoplasy within the following taxa:
- Eusiroidea (crustaceans and Amphipoda)[24]
- Urticaceae[25]
- Asteraceae[26]
- Polypodioideae (Selligueoid Ferns)[27]
- Ants[28]
- Merluccius capensis (cape hakes) [29]
- Tarantula spiders of the genus Bonnetina: nearly all morphological traits within this genus are homoplastic. Only sexual features were observed to not be homoplastic, suggesting that sexual selection may have been a driving force in the divergence of tarantulas.[30]
- Gharials, with homoplasy between Tomistoma and true crocodiles, and between Thoracosaurus and Gavialis.[31]
The occurrence of homoplasy can also be used to make predictions about evolution. Recent studies have used homoplasy to predict the possibility and the path of extraterrestrial evolution. For example, Levin et al. (2017) suggest that the development of eye-like structures is highly likely, due to its numerous, independently evolved incidences on earth.[16][32]
Homoplasy vs. evolutionary contingency
In his book Wonderful Life, Stephen Jay Gould claims that repeating the evolutionary process, from any point in time onward, would not produce the same results.[33] The occurrence of homoplasy is viewed by some biologists as an argument against Gould’s theory of evolutionary contingency. Powell & Mariscal (2015) argue that this disagreement is caused by an equivocation and that both the theory of contingency and homoplastic occurrence can be true at the same time.[34]
References
- Torres-Montúfar A, Borsch T, Ochoterena H (May 2018). "When Homoplasy Is Not Homoplasy: Dissecting Trait Evolution by Contrasting Composite and Reductive Coding". Systematic Biology. 67 (3): 543–551. doi:10.1093/sysbio/syx053. PMID 28645204.
- Stearns SC, Hoekstra RF (2005). Evolution: an introduction (2nd ed.). Oxford: Oxford University Press. ISBN 9780199255634.
- Hall AR, Colegrave N (March 2008). "Decay of unused characters by selection and drift". Journal of Evolutionary Biology. 21 (2): 610–7. doi:10.1111/j.1420-9101.2007.01473.x. PMID 18081745. S2CID 11165522.
- Reece JB, Urry LA, Cain ML, Wasserman SA, Minorsky PV, Jackson RB (2011). Campbell Biology (9th ed.). Pearson. ISBN 9780321739759.
- Sanderson MJ, Hufford L (1996). Homoplasy: The Recurrence of Similarity in Evolution. San Diego, CA: Academic Press, Inc. ISBN 0-12-618030-X.
- Silberfeld T, Leigh JW, Verbruggen H, Cruaud C, de Reviers B, Rousseau F (August 2010). "A multi-locus time-calibrated phylogeny of the brown algae (Heterokonta, Ochrophyta, Phaeophyceae): Investigating the evolutionary nature of the "brown algal crown radiation"". Molecular Phylogenetics and Evolution. 56 (2): 659–74. doi:10.1016/j.ympev.2010.04.020. PMID 20412862.
- de Queiroz A, Wimberger PH (February 1993). "The usefulness of behavior for phylogeny estimation: levels of homoplasy in behavioral and morphological characters". Evolution; International Journal of Organic Evolution. 47 (1): 46–60. doi:10.1111/j.1558-5646.1993.tb01198.x. PMID 28568085. S2CID 205778379.
- Lankester ER (1870). "On the use of the term homology in modern zoology, and the distinction between homogenetic and homoplastic agreements". Annals and Magazine of Natural History. 6 (31): 34–43. doi:10.1080/00222937008696201.
- Bailly A (1981-01-01). Abrégé du dictionnaire grec français. Paris: Hachette. ISBN 2010035283. OCLC 461974285.
- Bailly A. "Greek-french dictionary online". www.tabularium.be. Retrieved October 25, 2018.
- Holt JR, Judica CA (February 4, 2014). "Systematic Biology - Dictionary of Terms: Homoplasy". Archived from the original on July 1, 2017. Retrieved September 21, 2018.
- Archie JW (1989). "Homoplasy excess ratios: new indices for measuring levels of homoplasy in phylogenetic systematics and a critique of the consistency index". Systematic Biology. 38 (3): 253–269. doi:10.2307/2992286. JSTOR 2992286.
- Wake DB (September 1991). "Homoplasy: The Result of Natural Selection, or Evidence of Design Limitations?". The American Naturalist. 138 (3): 543–567. doi:10.1086/285234. S2CID 85043463.
- Hodin J (2000). "Plasticity and constraints in development and evolution". Journal of Experimental Zoology. 288 (1): 1–20. doi:10.1002/(SICI)1097-010X(20000415)288:1<1::AID-JEZ1>3.0.CO;2-7. PMID 10750048.
- Nevo E (1979). "Adaptive convergence and divergence of subterranean mammals". Annual Review of Ecology and Systematics. 10: 269–308. doi:10.1146/annurev.es.10.110179.001413.
- Wake DB, Wake MH, Specht CD (February 2011). "Homoplasy: from detecting pattern to determining process and mechanism of evolution". Science. 331 (6020): 1032–5. Bibcode:2011Sci...331.1032W. doi:10.1126/science.1188545. PMID 21350170. S2CID 26845473.
- Fong DW, Kane TC, Culver DC (1995). "Vestigialization and loss of nonfunctional characters". Annual Review of Ecology and Systematics. 26: 249–68. doi:10.1146/annurev.es.26.110195.001341.
- Jones R, Culver DC (May 1989). "Evidence for selection on sensory structures in a cave population of Gammarus minus (Amphipoda)". Evolution; International Journal of Organic Evolution. 43 (3): 688–693. doi:10.1111/j.1558-5646.1989.tb04267.x. PMID 28568387. S2CID 32245717.
- Skinner A, Lee MS (2009). "Body-form evolution in the scincid lizard Lerista and the mode of macroevolutionary transitions". Evolutionary Biology. 36: 292–300. doi:10.1007/s11692-009-9064-9. S2CID 42060285.
- Skinner A, Lee MS, Hutchinson MN (November 2008). "Rapid and repeated limb loss in a clade of scincid lizards". BMC Evolutionary Biology. 8: 310. doi:10.1186/1471-2148-8-310. PMC 2596130. PMID 19014443.
- Wiley EO, Lieberman BS (2011). Phylogenetics: Theory and Practice of Phylogenetic Systematics. Hoboken, NJ: John Wiley & Sons, Inc. ISBN 9780470905968.
- Schuh RT, Brower AV (2009). Biological Systematics: Principles and Applications. Ithaca, NY: Cornell University Press. ISBN 9780801462436.
- Felsenstein J (2004). Inferring phylogenies. Sinauer. ISBN 978-0878931774.
- Verheye ML, Martin P, Backeljau T, D'Udekem D'Acoz C (2015-12-22). "DNA analyses reveal abundant homoplasy in taxonomically important morphological characters of Eusiroidea (Crustacea, Amphipoda)". Zoologica Scripta. 45 (3): 300–321. doi:10.1111/zsc.12153. S2CID 86052388.
- Wu ZY, Milne RI, Chen CJ, Liu J, Wang H, Li DZ (2015-11-03). "Ancestral State Reconstruction Reveals Rampant Homoplasy of Diagnostic Morphological Characters in Urticaceae, Conflicting with Current Classification Schemes". PLOS ONE. 10 (11): e0141821. Bibcode:2015PLoSO..1041821W. doi:10.1371/journal.pone.0141821. PMC 4631448. PMID 26529598.
- Mejías JA, Chambouleyron M, Kim SH, Infante MD, Kim SC, Léger JF (2018-07-19). "Phylogenetic and morphological analysis of a new cliff-dwelling species reveals a remnant ancestral diversity and evolutionary parallelism in Sonchus (Asteraceae)". Plant Systematics and Evolution. 304 (8): 1023–1040. doi:10.1007/s00606-018-1523-2. S2CID 49873212.
- He L, Schneider H, Hovenkamp P, Marquardt J, Wei R, Wei X, Zhang X, Xiang Q (2018-05-09). "A molecular phylogeny of selligueoid ferns (Polypodiaceae): Implications for a natural delimitation despite homoplasy and rapid radiation". Taxon. 67 (2): 237–249. doi:10.12705/672.1.
- Schär S, Talavera G, Espadaler X, Rana JD, Andersen Andersen A, Cover SP, Vila R (2018-06-27). "Do Holarctic ant species exist? Trans-Beringian dispersal and homoplasy in the Formicidae". Journal of Biogeography. 45 (8): 1917–1928. doi:10.1111/jbi.13380. S2CID 51832848.
- Henriques R, von der Heyden S, Matthee CA (2016-03-28). "When homoplasy mimics hybridization: a case study of Cape hakes (Merluccius capensis and M. paradoxus)". PeerJ. 4: e1827. doi:10.7717/peerj.1827. PMC 4824878. PMID 27069785.
- Ortiz D, Francke OF, Bond JE (October 2018). "A tangle of forms and phylogeny: Extensive morphological homoplasy and molecular clock heterogeneity in Bonnetina and related tarantulas". Molecular Phylogenetics and Evolution. 127: 55–73. doi:10.1016/j.ympev.2018.05.013. PMID 29778724. S2CID 29152043.
- Lee MS, Yates AM (June 2018). "Tip-dating and homoplasy: reconciling the shallow molecular divergences of modern gharials with their long fossil record". Proceedings. Biological Sciences. 285 (1881): 20181071. doi:10.1098/rspb.2018.1071. PMC 6030529. PMID 30051855.
- Levin SR, Scott TW, Cooper HS, West SA (2017). "Darwin's aliens". International Journal of Astrobiology. 18: 1–9. doi:10.1017/S1473550417000362.
- Gould SJ (2000). Wonderful Life: The Burgess Shale and the Nature of History. London: Vintage Books. ISBN 9780099273455.
- Powell R, Mariscal C (December 2015). "Convergent evolution as natural experiment: the tape of life reconsidered". Interface Focus. 5 (6): 20150040. doi:10.1098/rsfs.2015.0040. PMC 4633857. PMID 26640647.