Horseshoe crab
Horseshoe crabs are marine and brackish water arthropods of the family Limulidae and the only living members of the order Xiphosura.[2][3] Despite their name, they are not true crabs or crustaceans: they are chelicerates, most closely related to arachnids such as spiders, ticks, and scorpions.[4][5][6]
| Limulidae Temporal range: | |
|---|---|
 | |
| Tachypleus gigas, one of the four extant species | |
 | |
| Jurassic-aged limulids. Crenatolimulus (A,B), Limulus (C), Mesolimulus (D,E) | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Subphylum: | Chelicerata |
| Order: | Xiphosura |
| Superfamily: | Limuloidea |
| Family: | Limulidae Leach, 1819[1][2] |
| Genera | |
|
See text | |
Horseshoe crabs live primarily in and around shallow coastal waters on soft, sandy or muddy bottoms. They are generally found in the intertidal zone at spring high tides.[7] They are eaten in some parts of Asia, and used as fishing bait, in fertilizer and in science (especially Limulus amebocyte lysate, which is used for the detection and quantification of bacterial endotoxins). In recent years, population declines have occurred as a consequence of coastal habitat destruction and overharvesting.[3] Tetrodotoxin may be present in one horseshoe crab species, Carcinoscorpius rotundicauda.[8]
The fossil record of Xiphosura goes back over 440 million years to the Ordovician period, with the oldest representatives of the modern family Limulidae dating to approximately 250 million years ago during the Early Triassic. As such, the extant forms have been described as "living fossils".[9] Some molecular analyses have placed Xiphosura within Arachnida, with a 2019 molecular analysis placing them as the sister group of Ricinulei.[10]
Taxonomy
The family name Limulidae comes from the genus Limulus, from the word limulus in Latin meaning "askance",[11] or "a little askew".[12]
Horseshoe crabs resemble crustaceans but belong to a separate subphylum of the arthropods, Chelicerata.[13] Horseshoe crabs are closely related to the extinct eurypterids (sea scorpions), which include some of the largest arthropods to have ever existed, and the two may be sister groups.[13][14] Other studies have placed eurypterids closer to the arachnids in a group called Merostomata.[15] The enigmatic Chasmataspidids are also thought to be closely related to the horseshoe crabs.[16] The earliest horseshoe crab fossils are found in strata from the Lower Ordovician period, roughly 480 million years ago.[17]
The Limulidae are the only recent family of the order Xiphosura, and contains all four living species of horseshoe crabs:[1][3][18]
- Carcinoscorpius rotundicauda, the mangrove horseshoe crab, found in South and Southeast Asia
- Limulus polyphemus, the Atlantic or American horseshoe crab, found along the Atlantic coast of the United States and the Southeast Gulf of Mexico
- Tachypleus gigas, the Indo-Pacific, Indonesian, Indian or southern horseshoe crab, found in South and Southeast Asia
- Tachypleus tridentatus, the Chinese, Japanese or tri-spine horseshoe crab, found in Southeast and East Asia
Genera
After Bicknell et al. 2021 and Lamsdell et al. 2020[19][20]
- Incertae sedis
- †Albalimulus? Bicknell & Pates, 2019[21] Ballagan Formation, Scotland, Early Carboniferous (Tournaisian) (Considered Xiphosura incertae sedis by Lamsdell, 2020[20])
- †Casterolimulus Holland, Erickson & O'Brien, 1975 Late Cretaceous (Maastrichtian) Fox Hills Formation, North Dakota, USA (Inconsistently placed in this family)
- †Heterolimulus gadeai Vıa & Villalta, 1966 Alcover Limestone Formation, Spain, Middle Triassic (Ladinian)
- †Limulitella Størmer, 1952 Middle-Upper Triassic, France, Germany, Tunisia, Russia
- †Sloveniolimulus Bicknell et al., 2019 Strelovec Formation, Slovenia Middle Triassic (Anisian)
- †Tarracolimulus Romero & Vıa Boada, 1977 Alcover Limestone Formation, Spain, Middle Triassic (Ladinian)
- †Victalimulus Riek & Gill, 1971 Lower Cretaceous (Aptian) Korumburra Group, NSW, Australia
- †Yunnanolimulus Zhang et al., 2009 Middle Triassic (Anisian), Guanling Formation, Yunnan, China
- †Mesolimulus Middle Triassic-Late Cretaceous England, Spain, Siberia, Germany, Morocco
- †Ostenolimulus Lamsdell et al. 2021[22] Early Jurassic (Sinemurian) Moltrasio Limestone, Italy
- †Volanalimulus Lamsdell, 2020[20] Early Triassic, Madagascar.
- Subfamily Limulinae Leach, 1819
- †Crenatolimulus Feldmann et al., 2011 Upper Jurassic (upper Tithonian) Kcynia Formation, Poland. Lower Cretaceous (Albian) Glen Rose Formation, Texas, USA
- Limulus O. F. Müller, 1785 Pierre Shale, United States, Late Cretaceous (Maastrichtian), Atlantic North America, Recent
- Subfamily Tachypleinae Pocock, 1902
- Carcinoscorpius Pocock, 1902, Asia, Recent
- Tachypleus Leach, 1819 Upper Cretaceous (Cenomanian) Haqel and Hjoula Konservat-Lagerstatten, Lebanon, Upper Eocene Domsen Sands, Germany, Asia, Recent
Cladogram after Lasmdell 2020.[20]
| Limulidae |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Anatomy

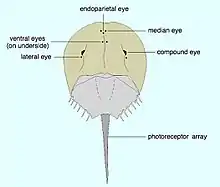
The entire body of the horseshoe crab is protected by a hard carapace. It has two compound lateral eyes, each composed of about 1,000 ommatidia, plus a pair of median eyes that are able to detect both visible light and ultraviolet light, a single parietal eye, and a pair of rudimentary lateral eyes on the top. The latter becomes functional just before the embryo hatches. Also, a pair of ventral eyes is located near the mouth, as well as a cluster of photoreceptors on the telson. Having relatively poor eyesight, the animals have the largest rods and cones of any known animal, about 100 times the size of humans,[23][24] and their eyes are a million times more sensitive to light at night than during the day.[25]
They use their chelicerae—a pair of small appendages—for moving food into the mouth. The next five pairs of appendages, the first of which are the pedipalps, are used for locomotion (ambulatory legs). The mouth is located in the center of the legs, whose bases are referred to as gnathobases, and have the same function as jaws and help grind up food.[26] In extant species and other fossil xiphosurans (e.g. Alanops[27]) their appendages are uniramous, but in other related non-xiphosurid euchelicerates such as Offacolus and Dibasterium the appendages are biramous.[28] The pedipalps on a male change shape on their terminal molt, becoming boxing glove-like claspers that are used for grasping the female during mating. The last pair of legs for both males and females are the main legs used for pushing when walking on the ocean floor. The remaining leg pairs have a weak claw at the tip.[29] Lost legs or the telson (tail) may slowly regenerate, and cracks in the body shell can heal.[30]
| External video | |
|---|---|
 | |
Behind its legs, the horseshoe crab has book gills, which exchange respiratory gases, and are also occasionally used for swimming.[31] As in other arthropods, a true endoskeleton is absent, but the body does have an endoskeletal structure made up of cartilaginous plates that support the book gills.
Growth
Females are about 20–30% larger than males.[37] The smallest species is C. rotundicauda and the largest is T. tridentatus.[38] On average, males of C. rotundicauda are about 30 centimetres (12 inches) long, including a tail (telson) that is about 15 cm (6 in), and their carapace (prosoma) is about 15 cm (6 in) wide.[39] Some southern populations (in the Yucatán Peninsula) of L. polyphemus are somewhat smaller, but otherwise this species is larger.[37]
In the largest species, T. tridentatus, females can reach as much as 79.5 cm (31+1⁄4 in) long, including their tail, and up to 4 kg (9 lb) in weight.[40] This is only about 10–20 cm (4–8 in) longer than the largest females of L. polyphemus and T. gigas, but roughly twice the weight.[41][42]
The juveniles grow about 33% larger with every molt until reaching adult size.[43] Atlantic horseshoe crabs molt in late July.
Diet
Horseshoe crabs are more often found on the ocean floor searching for worms and molluscs, which are their main food. They may also feed on crustaceans and even small fish.[44]
Breeding

.jpg.webp)
During the breeding season (spring and summer in the Northeast U.S.; year-round in warmer locations or when the full moon rises),[45] horseshoe crabs migrate to shallow coastal waters. The smaller male horseshoe crab clings to the back or opisthosoma of the larger female using specialized front claws and fertilizes the eggs as they are laid in the sand. Additional males called "satellite males" which are not attached to the female may surround the pair and have some success in fertilizing eggs.[46] Young female horseshoe crabs can be identified by the lack of mating scars.[47]
The female can lay between 60,000 and 120,000 eggs in batches of a few thousand at a time. The eggs may be inseminated within 20 to 30 minutes.[45] In L. polyphemus, the eggs take about two weeks to hatch; shore birds eat many of them before they hatch. The larvae molt six times during the first year and annually after the first 3 or 4 years.[48][49]
Natural breeding of horseshoe crabs in captivity has proven to be difficult. Some evidence indicates that mating takes place only in the presence of the sand or mud in which the horseshoe crab's eggs were hatched; it is not known with certainty what is in the sand that the crabs can sense or how they sense it.[50] Artificial insemination and induced spawning have been done on a relatively large scale in captivity, and eggs and juveniles collected from the wild are often raised to adulthood in captivity.[51][52]
In order to preserve and ensure the continuous supply of the horseshoe crab, a breeding centre was built in Johor, Malaysia where the crabs are bred and released back into the ocean in thousands once every two years. It is estimated to take around 12 years before they are suitable for consumption.[53]
Relationships with humans
Blood harvesting
Horseshoe crabs use hemocyanin to carry oxygen through their blood.[54] Their blood contains amebocytes, which play a similar role to the white blood cells of vertebrates in defending the organism against pathogens. Amebocytes from the blood of L. polyphemus are used to make Limulus amebocyte lysate (LAL), which is used for the detection of bacterial endotoxins in medical applications.[55] There is a high demand for the blood, the harvest of which involves collecting and bleeding the animals, and then releasing them back into the sea. Most of the animals survive the process; mortality is correlated with both the amount of blood extracted from an individual animal, and the stress experienced during handling and transportation.[56] Estimates of mortality rates following blood harvesting vary from 3–15%[57][58] to 10–30%.[59][60][61] Approximately 500,000 Limulus are harvested annually for this purpose.[62] Declining horseshoe crab populations on the East Coast of the United States endanger certain bird species which feed upon their eggs.[58]
Bleeding may also prevent female horseshoe crabs from being able to spawn[58] or decrease the number of eggs they are able to lay. Up to 30% of an individual's blood is removed, according to the biomedical industry, although NPR reported that it "can deplete them of more than half their volume of blue blood."[58] The horseshoe crabs spend between one and three days away from the ocean before being returned. As long as the gills stay moist, they can survive on land for four days.[63] Some scientists are skeptical that certain companies return their horseshoe crabs to the ocean at all, instead suspecting them of selling the horseshoe crabs as fishing bait.[64]
The harvesting of horseshoe crab blood in the pharmaceutical industry is in decline. In 1986, Kyushu University researchers discovered that the same test could be achieved by using isolated Limulus clotting factor C (rFC), an enzyme found in LAL, as by using LAL itself.[65] Jeak Ling Ding, a National University of Singapore researcher, patented a process for manufacturing rFC; on 8 May 2003, synthetic isolated rFC made via her patented process became available for the first time.[66] Industry at first took little interest in the new product, however, as it was patent-encumbered, not yet approved by regulators, and sold by a single manufacturer, Lonza Group. In 2013, however, Hyglos GmbH also began manufacturing its own rFC product. This, combined with the acceptance of rFC by European regulators, the comparable cost between LAL and rFC, and support from Eli Lilly and Company, which has committed to use rFC in lieu of LAL,[58] is projected to all but end the practice of blood harvesting from horseshoe crabs.[67]
In December 2019, a report of the US Senate which encouraged the Food and Drug Administration to "establish processes for evaluating alternative pyrogenicity tests and report back [to the Senate] on steps taken to increase their use" was released;[68] PETA backed the report.[69]
In June 2020, it was reported that U.S. Pharmacopeia had declined to give rFC equal standing with horseshoe crab blood.[70] Without the approval for the classification as an industry standard testing material, U.S. companies will have to overcome the scrutiny of showing that rFC is safe and effective for their desired uses, which may serve as a deterrent for usage of the horseshoe crab blood substitute.[71]
Vaccine research and development during the COVID-19 pandemic has added additional "strain on the American horseshoe crab."[72]
In 2023, the U.S. Fish and Wildlife Service halted the harvesting of horseshoe crabs in the Cape Romain National Wildlife Refuge, South Carolina, from March 15 to July 15 to aid their reproduction. This decision was influenced by the importance of horseshoe crab eggs as a food source for migratory birds and the ongoing use of horseshoe crabs for bait and their blood in medical products. The ban supports the conservation goals of the refuge, spanning 66,000 acres (26,700 hectares) of marshes, beaches, and islands near Charleston.[73]
Fishery
Horseshoe crabs are used as bait to fish for eels (mostly in the United States) and whelk, or conch. Nearly 1 million (1,000,000) crabs a year are harvested for bait in the United States, dwarfing the biomedical mortality. However, fishing with horseshoe crab was banned indefinitely in New Jersey in 2008 with a moratorium on harvesting to protect the red knot, a shorebird which eats the crab's eggs.[74] A moratorium was restricted to male crabs in Delaware, and a permanent moratorium is in effect in South Carolina.[75] The eggs are eaten in parts of Southeast Asia, Johor and China.[76]
A low horseshoe crab population in the Delaware Bay is hypothesized to endanger the future of the red knot. Red knots, long-distance migratory shorebirds, feed on the protein-rich eggs during their stopovers on the beaches of New Jersey and Delaware.[77] An effort is ongoing to develop adaptive-management plans to regulate horseshoe crab harvests in the bay in a way that protects migrating shorebirds.[78]
Culinary use
The population of T. gigas in Indonesia and Malaysia has decreased dramatically in the past decade. The harvesting of T. gigas is largely used to supply Thailand with primarily female T. gigas, which is considered a local delicacy. This female biased harvesting has led to an unbalanced sex ratio in the wild, which also contributes to its declining population in the area.[79]
Conservation status
Development along shorelines is dangerous to horseshoe crab spawning, limiting available space and degrading habitat. Bulkheads can block access to intertidal spawning regions as well.[80]
Because of the destruction of habitat and shoreline development, use in fishing, plastic pollution, status as a culinary delicacy in some areas, and use for scientific research and advancements, the horseshoe crab is facing down endangered and extinct statuses. One species, T. tridentatus, has already been declared extirpated from Taiwan. Facing a greater than 90% population decrease in T. tridentatus juveniles, it is suspected that Hong Kong will be the next to declare the horseshoe crab extirpated from its area. The species is listed as endangered on the IUCN Red List, specifically because of overexploitation and loss of critical habitat leading to a steep decline in population size.[79]
References
- Sekiguchi K (1988). Biology of Horseshoe Crabs. Science House. ISBN 978-4-915572-25-8.
- "Limulidae Leach, 1819". World Register of Marine Species. Flanders Marine Institute. 2023. Retrieved 17 January 2023.
- Vestbo, Stine; Obst, Matthias; Quevedo Fernandez, Francisco J.; Intanai, Itsara; Funch, Peter (May 2018). "Present and Potential Future Distributions of Asian Horseshoe Crabs Determine Areas for Conservation". Frontiers in Marine Science. 5 (164): 1–16. doi:10.3389/fmars.2018.00164.
- Chliboyko, J. (April 2008). "Crabby Ancestors". Canadian Geographic. p. 25.
- Lamerato, Amanda (2001). "Limulus polyphemus". Animal Diversity Web. University of Michigan Museum of Zoology. Retrieved 17 January 2023.
- Sharma, Prashant P.; Ballesteros, Jesús A. (2019). "A Critical Appraisal of the Placement of Xiphosura (Chelicerata) with Account of Known Sources of Phylogenetic Error". Systematic Biology. 68 (6): 896–917. doi:10.1093/sysbio/syz011. PMID 30917194.
- Smith DR, Beekey MA, Brockmann HJ, King TL, Millard MJ, Zaldívar-Rae JA (2016). "Limulus polyphemus". IUCN Red List of Threatened Species. IUCN. 2016: e.T11987A80159830. doi:10.2305/IUCN.UK.2016-1.RLTS.T11987A80159830.en. Retrieved 12 March 2019.
- Kungsuwan A, Suvapeepan S, Suwansakornkul P, Shida Y, Suvapeepan S, Suwansakornkul P, Hashimoto K (1987). "Tetrodotoxin in the Horseshoe Crab Carcinoscorpius rotundicauda Inhabiting Thailand" (PDF). Nippon Suisan Gakkaishi. 53 (2): 261–266. doi:10.2331/suisan.53.261.
- Lamsdell, James C.; McKenzie, Scott C. (1 August 2015). "Tachypleus syriacus (Woodward)—a sexually dimorphic Cretaceous crown limulid reveals underestimated horseshoe crab divergence times". Organisms Diversity & Evolution. 15 (4): 681–693. doi:10.1007/s13127-015-0229-3. S2CID 15196244.
- Ballesteros JA, Sharma PP (November 2019). "A Critical Appraisal of the Placement of Xiphosura (Chelicerata) with Account of Known Sources of Phylogenetic Error". Systematic Biology. 68 (6): 896–917. doi:10.1093/sysbio/syz011. PMID 30917194.
- Lewis, Charlton T.; Short, Charles (1879). "līmŭlus". A Latin Dictionary. Perseus Digital Library.
- Heard, Willie (Fall 2001). "Coast" (PDF). Project Oceanography: 81–91. Archived from the original (PDF) on 19 February 2017. Retrieved 5 July 2008.
- Garwood RJ, Dunlop J (13 November 2014). "Three-dimensional reconstruction and the phylogeny of extinct chelicerate orders". PeerJ. 2: e641. doi:10.7717/peerj.641. PMC 4232842. PMID 25405073.
- Clarke JM, Ruedemann R. The Eurypterida of New York.
- Weygoldt P, Paulus HF (1979). "Untersuchungen zur Morphologie, Taxonomie und Phylogenie der Chelicerata". Zeitschrift für zoologische Systematik und Evolutionsforschung. 17 (2): 85–116, 177–200. doi:10.1111/j.1439-0469.1979.tb00694.x.
- Garwood RJ, Dunlop JA, Knecht BJ, Hegna TA (April 2017). "The phylogeny of fossil whip spiders". BMC Evolutionary Biology. 17 (1): 105. doi:10.1186/s12862-017-0931-1. PMC 5399839. PMID 28431496.
- Bicknell R, Pates S (9 July 2020). "Pictorial Atlas of Fossil and Extant Horseshoe Crabs, With Focus on Xiphosurida". Frontiers in Earth Science. 8: 98. Bibcode:2020FrEaS...8...98B. doi:10.3389/feart.2020.00098.
- "About the Species". The Horseshoe Crab. Ecological Research & Development Group. 2003. Retrieved 17 January 2023.
- Bicknell RD, Błażejowski B, Wings O, Hitij T, Botton ML (March 2021). Zhang XG (ed.). "Critical re‐evaluation of Limulidae uncovers limited Limulus diversity". Papers in Palaeontology. 7 (3): 1525–1556. doi:10.1002/spp2.1352. S2CID 233783546.
- Lamsdell JC (2020-12-04). "The phylogeny and systematics of Xiphosura". PeerJ. 8: e10431. doi:10.7717/peerj.10431. PMC 7720731. PMID 33335810.
- Bicknell RD, Pates S (November 2019). "Xiphosurid from the Tournaisian (Carboniferous) of Scotland confirms deep origin of Limuloidea". Scientific Reports. 9 (1): 17102. Bibcode:2019NatSR...917102B. doi:10.1038/s41598-019-53442-5. PMC 6863854. PMID 31745138.
- Lamsdell JC, Teruzzi G, Pasini G, Garassino A (2021-04-29). "A new limulid (Chelicerata, Xiphosurida) from the Lower Jurassic (Sinemurian) of Osteno, NW Italy". Neues Jahrbuch für Geologie und Paläontologie - Abhandlungen. 300 (1): 1–10. doi:10.1127/njgpa/2021/0974. ISSN 0077-7749. S2CID 234814276.
- Anatomy: Vision – The Horseshoe Crab
- "Horseshoe Crabs, Limulus polyphemus". MarineBio.org. 18 May 2017.
- Palumbi SR, Palumbi AR (23 February 2014). The Extreme Life of the Sea. Princeton University Press. ISBN 9781400849932 – via Google Books.
- "Anatomy: Bottom View". The Horseshoe Crab.
- Racheboeuf, Patrick R.; Vannier, Jean; Anderson, Lyall I. (2002). "A New Three-Dimensionally Preserved Xiphosuran Chelicerate from the Montceau-Les-Mines Lagerstatte (Carboniferous, France)". Palaeontology. 45 (1): 125–147. doi:10.1111/1475-4983.00230. ISSN 0031-0239. S2CID 129150998.
- Briggs, D. E.; Siveter, D. J.; Siveter, D. J.; Sutton, M. D.; Garwood, R. J.; Legg, D. (2012). "Silurian horseshoe crab illuminates the evolution of arthropod limbs". Proceedings of the National Academy of Sciences of the United States of America. 109 (39): 15702–15705. Bibcode:2012PNAS..10915702B. doi:10.1073/pnas.1205875109. PMC 3465403. PMID 22967511.
- "Anatomy: Appendages". The Horseshoe Crab.
- Castillo Y, Garabedian LA (26 April 2007). Limb Regeneration in Horseshoe Crabs (PDF) (B.S. thesis). Worcester Polytechnical Institute.
- Person P, Philpott DE (May 1969). "The biology of cartilage. I. Invertebrate cartilages: Limulus gill cartilage". Journal of Morphology. 128 (1): 67–93. doi:10.1002/JMOR.1051280104. S2CID 86239230.
- Manton SM (1977) The Arthropoda: habits, functional morphology, and evolution Page 57, Clarendon Press.
- Shuster CN, Barlow RB and Brockmann HJ (Eds.) (2003) The American Horseshoe Crab Pages 163–164, Harvard University Press. ISBN 9780674011595.
- Vosatka, ed. (1970). "Observations on the Swimming, Righting, and Burrowing Movements of Young Horseshoe Crabs, Limulus Polyphemus". The Ohio Journal of Science. 70 (5): 276–283. hdl:1811/5558.
- Battelle BA (December 2006). "The eyes of Limulus polyphemus (Xiphosura, Chelicerata) and their afferent and efferent projections". Arthropod Structure & Development. 35 (4): 261–274. doi:10.1016/j.asd.2006.07.002. PMID 18089075.
- Barlow RB (2009) "Vision in horseshoe crabs" Pages 223–235 in JT Tanacredi, ML Botton and D Smith, Biology and Conservation of Horseshoe Crabs, Springer. ISBN 9780387899589.
- Zaldívar-Rae J, Sapién-Silva RE, Rosales-Raya M, Brockmann HJ (2009). "American horseshoe crabs, Limulus polyphemus, in México: open possibilities". In J.T. Tanacredi, M.L. Botton, D.R. Smith (eds.). Biology and Conservation of Horseshoe Crabs. Springer. pp. 97–113. ISBN 9780387899589.
- "About the Species". The Horseshoe Crab. Retrieved 26 June 2018.
- Srijaya TC, Pradeep PJ, Mithun S, Hassan A, Shaharom F, Chatterji A (2010). "A New Record on the Morphometric Variations in the Populations of Horseshoe Crab (Carcinoscorpius rotundicauda Latreille) Obtained from Two Different Ecological Habitats of Peninsular Malaysia". Our Nature. 8 (1): 204–211. doi:10.3126/on.v8i1.4329.
- Manca A, Mohamad F, Ahmad A, Sofa MF, Ismail N (2017). "Tri-spine horseshoe crab, Tachypleus tridentatus (L.) in Sabah, Malaysia: the adult body sizes and population estimate". Journal of Asia-Pacific Biodiversity. 10 (3): 355–361. doi:10.1016/j.japb.2017.04.011.
- "Horseshoe Crab (Limulus polyphemus)". WAZA. Archived from the original on 3 July 2017. Retrieved 26 June 2018.
- Jawahir AR, Samsur M, Shabdin ML, Rahim KA (2017). "Morphometric allometry of horseshoe crab, Tachypleus gigas at west part of Sarawak waters, Borneo, East Malaysia". AACL Bioflux. 10 (1): 18–24.
- Cartwright-Taylor L, Lee J, Hsu CC (2009). "Population structure and breeding pattern of the mangrove horseshoe crab Carcinoscorpius rotundicauda in Singapore" (PDF). Aquatic Biology. 8 (1): 61–69. doi:10.3354/ab00206.
- "Horseshoe Crabs". MarineBio Conservation Society. 2017-05-18. Retrieved 2022-01-23.
- Mohamad Faizul Mat Isa (16 January 2021). "Belangkas : Potensi dan Masa Depan" [Horseshoe crabs: their potential and future]. Utusan Borneo (in Malay). Retrieved 17 January 2023 – via Universiti Putra Malaysia.
- "Facts About Horseshoe Crabs and FAQ". Florida Fish and Wildlife Conservation Commission. Retrieved 19 January 2020.
- "The Horseshoe Crab, limulus polyphemus: 200 Millions Years of Existence, 100 Years of study". 2002. Retrieved 3 February 2020.
- "The Rabbit and the Horse Shoe Crab". 2014-09-23. Retrieved 20 December 2016.
- "Molt". The Horseshoe Crab.
- Funkhouser D (April 15, 2011). "Crab love nest". Scientific American. 304 (4): 29. Bibcode:2011SciAm.304d..29F. doi:10.1038/scientificamerican0411-29.
- Chen Y, Lau CW, Cheung SG, Ke CH, Shin PK (2010). "Enhanced growth of juvenile Tachypleus tridentatus (Chelicerata: Xiphosura) in the laboratory: a step towards population restocking for conservation of the species". Aquatic Biology. 11: 37–40. doi:10.3354/ab00289.
- Carmichael RH, Brush E (2012). "Three decades of horseshoe crab rearing: A review of conditions for captive growth and survival". Reviews in Aquaculture. 4 (1): 32–43. doi:10.1111/j.1753-5131.2012.01059.x.
- "Horseshoe crab business still a hit despite pandemic". The Star. 13 December 2020. Retrieved 18 July 2021.
- "Anxious Arthropods". Ohio History Org. 2014-09-25. Archived from the original on 2020-03-29. Retrieved 2020-03-29.
- "The horseshoe crab and public health". HorseshoeCrab.org.
- Hurton L (2003). Reducing post-bleeding mortality of horseshoe crabs (Limulus polyphemus) used in the biomedical industry (PDF) (M.Sc. thesis). Virginia Polytechnic Institute and State University. hdl:10919/36231. Archived from the original on 2013-06-22. Retrieved 2020-09-20.
- "Crash: A Tale of Two Species – The Benefits of Blue Blood – Nature – PBS". PBS. 10 June 2008.
- Eisner, Chiara (2023-06-10). "Coastal biomedical labs are bleeding more horseshoe crabs with little accountability". NPR. Retrieved 2023-06-10.
- The Blood Harvest The Atlantic, 2014.
- Carmichael RH, Botton ML, Shin PK, Cheung SG, eds. (2015). Changing Global Perspectives on Horseshoe Crab Biology, Conservation and Management. Springer International Publishing.
- Chesler C. "Medical Labs May Be Killing Horseshoe Crabs". Scientific American. Retrieved 10 May 2018.
- Chesler C. "The Blood of the Crab". Popular Mechanics. No. 13 April 2017. Retrieved 16 April 2017.
- The Horseshoe Crab - US Fish and Wildlife Service
- Chesler C (June 9, 2016). "Medical Labs May Be Killing Horseshoe Crabs". Scientific American. Retrieved 2017-11-03.
- Iwanaga S, Morita T, Miyata T, Nakamura T, Aketagawa J (1986-08-01). "The hemolymph coagulation system in invertebrate animals". Journal of Protein Chemistry. 5 (4): 255–268. doi:10.1007/bf01025424. ISSN 0277-8033. S2CID 84664449.
- "PyroGene : Licensing Success". National University of Singapore. 8 May 2003. Retrieved 2018-09-01.
- Zhang S (2018-05-09). "The Last Days of the Blue-Blood Harvest". The Atlantic. Retrieved 2018-09-01.
- "S. Rept. 116-110 - AGRICULTURE, RURAL DEVELOPMENT, FOOD AND DRUG ADMINISTRATION, AND RELATED AGENCIES APPROPRIATIONS BILL, 2020". United States Congress. Retrieved 2020-01-18.
- "PETA Statement: U.S. Spending Bill". PETA. 2019-12-20. Retrieved 2020-01-18.
- Fox, Alex. "The Race for a Coronavirus Vaccine Runs on Horseshoe Crab Blood". Smithsonian Magazine. Retrieved 2020-06-09.
- "Drugs standards group nixes plan to kick pharma's crab blood habit". Reuters. 2020-05-30. Retrieved 2020-06-09.
- Iovenko C (17 December 2021). "Horseshoe crabs are in danger because everyone wants their blood". theverge.com. Archived from the original on 18 December 2021.
- Patrick Whittle (August 10, 2023). "Harvest of horseshoe crabs, needed for blue blood, stopped during spawning season in national refuge". AP News. Retrieved August 12, 2023.
- "N.J. law protects horseshoe crabs". UPI. 25 March 2008. Retrieved 27 July 2018.
- "Horseshoe crab". SC DNR species gallery. Archived from the original on March 31, 2016. Retrieved 2011-06-06.
- 大西一實. "Vol.56 食うか食われるか?". あくあは〜つ通信. Archived from the original on 2003-08-13. Retrieved 2008-04-18.
- "Red knots get to feast on horseshoe crab eggs". Environment News Service. March 26, 2008. Retrieved 2011-01-19.
- "Critter Class Hodge Podge (Horseshoe crabs and Wooly Bears)" (PDF). The Wildlife Center. October 26, 2011. Retrieved 2015-03-09.
- John A, Shin PK, Botton ML, Gauvry G, Cheung SG, Laurie K (2021-01-01). "Conservation of Asian horseshoe crabs on spotlight". Biodiversity and Conservation. 30 (1): 253–256. doi:10.1007/s10531-020-02078-3. PMC 7651794. PMID 33191986.
- "Conservation". ERDG. Retrieved 2016-05-19.
Further reading
- Chisholm H, ed. (1911). "King-Crab". Encyclopædia Britannica (11th ed.). Cambridge University Press.
External links
 Media related to Limulidae at Wikimedia Commons
Media related to Limulidae at Wikimedia Commons- When the Horseshoe Crabs Are Gone, We’ll Be in Trouble. New York Times 2023
- LAL Update
- Science Friday Video: horseshoe crab season
- Horseshoe crab at the Smithsonian Ocean Portal
- The Horseshoe Crab – Medical Uses; The Ecological Research & Development Group (ERDG)
- RedKnot.org links to shorebird recovery sites, movies, events & other info on Red Knot rufa & horseshoe crabs.
- Crab Bleeders Article about the men who bleed horseshoe crabs for science.
- Day time mating of horseshoe crabs in Maine
- Sarah Zhang, The Last Days of the Blue-Blood Harvest, The Atlantic, May 9, 2018