Hydrazine nitrate
Hydrazine nitrate is an inorganic compound with the chemical formula N2H4·HNO3. It has usage in liquid explosives as an oxidizer. It exists in two crystalline forms, stable α-type and unstable β-type. The former is usually used in explosives.[1] Its solubility is small in alcohols but large in water and hydrazine. It has strong hygroscopicity, only slightly lower than ammonium nitrate.
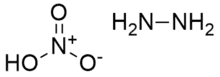 | |
| Names | |
|---|---|
| Other names
hydrazinium nitrate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| Molar mass | 95.02 |
| Appearance | Clear liquid |
| Density | 1.64 g/cm3 |
| Melting point | 72°C |
| Soluble in water | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Hydrazine nitrate has a good thermal stability. Its weight loss rate at 100 °C is slower than that of ammonium nitrate. Its explosion point is 307 °C (50% detonation) and explosion heat is about 3.829 MJ/kg. Because it has no carbon elements, the detonation products are not solid and their average molecular weight is small.
Production
Hydrazine nitrate is produced by the reaction of hydrazine and nitric acid:[2]
- N2H4 + HNO3 → N2H5NO3
References
- Liu, Jiping (2015). Liquid Explosives. Springer. p. 6. ISBN 9783662458464.
- D. G. Karraker (1981). "Cu(II) - Catalyzed Hydrazine Reduction of Ferric Nitrate" (PDF). United States Department of Energy. doi:10.2172/5658572.
{{cite journal}}: Cite journal requires|journal=(help)