Hydrogel encapsulation of quantum dots
The behavior of quantum dots (QDs) in solution and their interaction with other surfaces is of great importance to biological and industrial applications, such as optical displays, animal tagging, anti-counterfeiting dyes and paints, chemical sensing, and fluorescent tagging. However, unmodified quantum dots tend to be hydrophobic, which precludes their use in stable, water-based colloids. Furthermore, because the ratio of surface area to volume in a quantum dot is much higher than for larger particles, the thermodynamic free energy associated with dangling bonds on the surface is sufficient to impede the quantum confinement of excitons. Once solubilized by encapsulation in either a hydrophobic interior micelle or a hydrophilic exterior micelle, the QDs can be successfully introduced into an aqueous medium, in which they form an extended hydrogel network. In this form, quantum dots can be utilized in several applications that benefit from their unique properties, such as medical imaging and thermal destruction of malignant cancers.[1]
Quantum dots
Quantum dots (QDs) are nano-scale semiconductor particles on the order of 2-10 nm in diameter. They possess electrical properties between those of bulk semi-conductors and individual molecules, as well as optical characteristics that make them suitable for applications where fluorescence is desirable, such as medical imaging. Most QDs synthesized for medical imaging are in the form of CdSe(ZnS) core(shell) particles. CdSe QDs have been shown to possess optical properties superior to organic dyes.[2] The ZnS shell has a two-fold effect:
- to interact with dangling bonds that would otherwise result in particle aggregation, loss of visual resolution, and impedance of quantum confinement effects
- to further increase the fluorescence of the particles themselves.[3]
Problems with CdSe(ZnS) quantum dots
Despite their potential for use as contrast agents for medical imaging techniques, their use in vivo is hindered by the cytotoxicity of Cadmium. To address this issue, methods have been developed to “wrap” or “encapsulate” potentially-toxic QDs in bio-inert polymers to facilitate use in living tissue. While Cd-free QDs are commercially available, they are unsuitable for use as a substitute for organic contrasts.[4] Another issue with CdSe(ZnS) nanoparticles is significant hydrophobicity, which hinders their ability to enter solution with aqueous media, such as blood or spinal fluid. Certain hydrophilic polymers could be used to render the dots water-soluble.
Synthesizing the encapsulant polymer
Rf-PEG synthesis
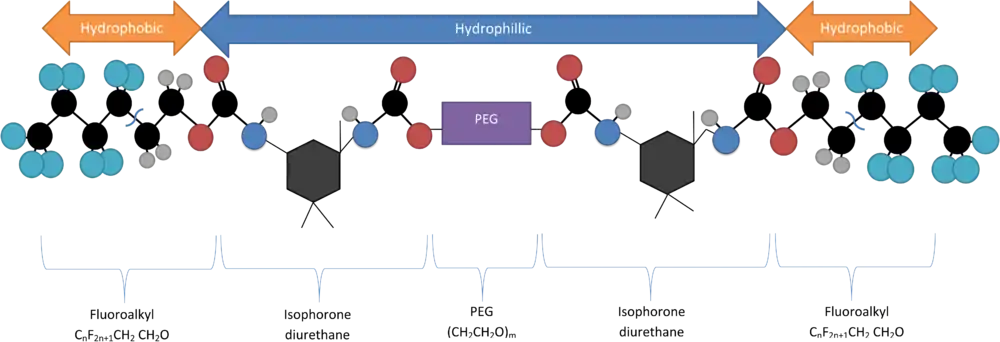
One notable quantum dot encapsulation technique involves utilizing a double fluoroalkyl-ended polyethylene glycol molecule (Rf-PEG) as a surfactant, which will spontaneously form micellular structures at its critical micelle concentration (CMC). The critical micelle concentration of the Rf-PEG depends on the length of the PEG portion of the polymer. This molecule consists of a hydrophilic PEG backbone with two hydrophilic terminal groups (CnF2n+1-CH2CH2O) attached via isophorone diurethane.[5] It is synthesized by dehydrating a solution of 1,3-dimethyl-5-fluorouracil and PEG, mixing them in the presence of heavy water (D2O) via a sonicator to combine then.[6]

Micellization

At the appropriate Krafft temperature and critical micelle concentration these molecules will form individual tear-drop loops, where the hydrophobic ends are attracted to one another, to other molecules, and also to the similarly hydrophobic QDs. This forms a loaded micelle with a hydrophilic outer shell and a hydrophobic core.[6]
When encapsulating hydrophobes in this way it is important to ensure the particle size is appropriate for the PEG backbone being utilized, as the number of PEG mer units (generally with a MW of 6K or 10K Daltons) determines the maximum particle size that can be successfully contained at the core of the micelle.
To determine the average diameter, D, of the QDs, the following empirical equation is used:
Where
- is the diameter of the CdSe QD in nm
- is the wavelength of the first absorption peak in nm
Role of ZnS shell
It is during encapsulation that the ZnS shell plays an especially important role, in that it helps prevent the agglomeration of CdSe particles that had no shell by occupying the previously mentioned bonds on the dot's surface; however, clumping can still occur through secondary forces that arise from common hydrophobicity. This can result in multiple particles within each micelle, which may negatively impact overall resolution. For this reason multiple combinations of PEG chain length and particle diameter are necessary to achieve optimal imaging properties.
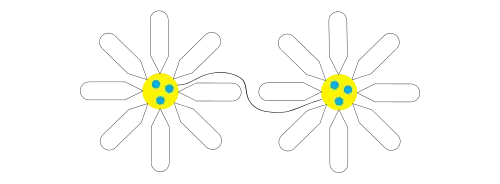
Hydrogel network
After initial encapsulation the remaining molecules form connections between the individual micelles to form a network within the aqueous media called a hydrogel, creating a diffuse and relatively constant concentration of the encapsulated particle within the gel. The formation of hydrogels is a phenomenon observed in superabsorbent polymers, or "slush powders," in which the polymer, often in the form of a powder, absorbs water, becoming up to 99% liquid and 30-60 times larger in size.[7]
Stokes-Einstein equation
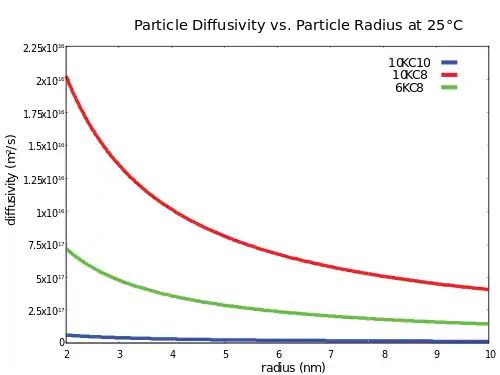
The diffusivity of spherical particles in a suspension is approximated by the Stokes–Einstein equation:[6]
- where
- is the temperature
- is the particle radius
- is the Boltzmann constant
- is the hydrogel viscosity
Typical Rf-PEG hydrogel diffusivities for 2 nm quantum dots are on the order of 10−16 m2/s, so suspensions of quantum dots tend to be very stable. Hydrogel viscosity can be determined by using rheological techniques.
Micelle rheology
When encapsulating hydrophobic or potentially toxic materials it is important that the encapsulant remain intact while inside the body. Studying the rheological properties of the micelles permits identification and selection of the polymer that is most appropriate for use in long-term biological applications. Rf-PEG exhibits superior rheological properties when used in vivo.
Importance of Polymer Length
The properties of the polymer are influenced by the chain length. The correct chain length ensures that the encapsulant is not released over time. Avoiding the release of QDs and other toxic particles is critical to prevent unintentional cell necrosis in patients. The length of the polymer is controlled by two factors:
- Weight of the PEG backbone in Daltons, represented by #K (thousands of Daltons)
- Length of the hydrophobic ends, denoted by the number of carbon atoms in the terminal group (C#).
Increasing the PEG length increases the solubility of the polymer. However, if the PEG chain is too long the micelle will become unstable. It has been observed that a stable hydrogel can only be formed with PEG backbones weighing between six and ten thousand Daltons.[8]
On the other hand, increasing the length of the hydrophobic terminal groups decreases aqueous solubility. For a given PEG weight, if the hydrophobe is too short the polymer will just dissolve into the solution, and if it is too long the polymer won't dissolve at all. Generally, two end groups result in the highest conversion into micelles (91%):[8]
Maxwell fluid
At molecular weights between 6 thousand and 10 thousand Daltons the Rf-PEG hydrogel acts as a Maxwell material, which means the fluid has both viscosity and elasticity. This is determined by measuring the plateau modulus, the elastic modulus for a viscoelastic polymer is constant or "relaxed" when deformed, at a range of frequencies via oscillatory rheology.[9][10] Plotting the first- vs second-order integrals of the modulus values, a Cole-Cole plot is obtained, which, when fitted to a Maxwell model, provides the following relationship:
Where
- is the plateau modulus
- is the oscillation frequency in radians per second
Mechanical properties of common Rf-PEG molecules
Based on the Maxwellian behavior of the hydrogel and observations of erosion via surface plasmon resonance (SPR), the following data results for 3 common Rf-PEG types at their specified concentrations:[11][12]
| cellpadding = "3" cellspacing = "0" | |||
| 6.8 | 6.5 | 11.0 | |
| 1.2 | 0.029 | 0.023 | |
| 14.4 | 18.5 | 56.1 | |
| 18 | 0.53 | 1.5 | |
| 94 | 94 | 89 |
XKCY denotes X thousand Daltons of molecular mass and Y carbon atoms.
These values can give us information on the degree of entanglement (or degree of cross linking, depending on what polymer is being considered). In general, higher degrees of entanglement leads to higher time required for the polymer to return to the undeformed state or relaxation times.
Applications
Hydrogel encapsulation of the QDs opens up a new range of applications, such as:
- Biosensors
- Enzymes and other bio-active molecules serve as biorecognition units while QDs serve as signalling units. By adding enzymes to the QD hydrogel network both units can be combined to form a biosensor. The enzymatic reaction that detects a particular molecule causes the fluoresce of QDs to be quenched. In this way, the location of molecules of interest can be observed.[13]
- Cell Influence and Imaging
- Adding iron oxide nanoparticles to the QD micelles allows them to be fluorescent and magnetic. These micelles can be moved in a magnetic field to create concentration gradients that will influence a cell's processes.[14]
- Gold Hyperthermia
- When excited by high energy radiation, such as with a laser, gold nanoparticles emit a thermal field. This phenomenon can be used as a form of hyperthermia therapy to destroy malignant cancers without damaging surrounding tissues. When combined with QDs in a hydrogel this could facilitate real-time monitoring of the tumor treatment.[15]
See also
References
- Glazer, ES; SA Curley (July 2010). "Radiofrequency field-induced thermal cytotoxicity in cancer cells treated with fluorescent nanoparticles". Cancer. 116 (13): 3285–93. doi:10.1002/cncr.25135. PMC 2928886. PMID 20564640.
- Resch-Genger, Ute; Grabolle; Cavaliere-Jaricot; Nitschke; Nann (August 2008). "Quantum dots versus organic dyes as fluorescent labels". Nature Methods. 5 (9): 763–775. doi:10.1038/nmeth.1248. PMID 18756197. S2CID 9007994.
- Angell, Joshua. "Synthesis and Characterization of CdSe-ZnS Core-Shell Quantum Dots".
{{cite journal}}: Cite journal requires|journal=(help) - Jin, Shan; Yanxi Hu; Zhanjun Gu; Lei Liu; Hai-Chen Wu (July 2011). "Application of Quantum Dots in Biological Imaging". Journal of Nanomaterials. 2011: 1–13. doi:10.1155/2011/834139.
- Lundberg, D.J.; R.G. Brown; J.E. Glass; R.R. Eley (1994). "Synthesis, Characterization, and Solution Rheology of Model Hydrophobically-Modified, Water-Soluble Ethoxylated Urethanes". Langmuir. 10 (9): 3027–3034. doi:10.1021/la00021a028.
- Mathias, Errol V.; Julia Aponte; Julia A. Kornfield; Yong Ba (October 2010). "Properties of Small Molecular Drug Loading and Diffusion in a Fluorinated PEG Hydrogel Studied by 1H Molecular Diffusion NMR and 19F Spin Diffusion NMR". Colloid and Polymer Science. 288 (18): 1655–1663. doi:10.1007/s00396-010-2304-9. PMC 2982959. PMID 21170115.
- Horie, K, et al., 890.
- Tae, Giyoong; Julia A. Kornfield; Jeffry A. Hubbell; Diethelm Johannsmann; Thieo E. Hogen-Esch (May 2001). "Hydrogels with Controlled, Surface Erosion Characteristics from Self-Assembly of Fluoroalkyl-Ended Poly(ethylene glycol)". Macromolecules. 34 (18): 6409–6419. Bibcode:2001MaMol..34.6409T. doi:10.1021/ma0107511.
- Wyss, Hans; Ryan J. Larson; David A. Weitz (2007). "Oscillatory Rheology: Measuring the Viscoelastic Behaviour of Soft Materials" (PDF). G.I.T. Laboratory. 3 (4): 68–70.
- Rubinstein, M.; A.V. Dobrynin (1997). Trends in Polymer Science. 5 (6): 181.
{{cite journal}}: Missing or empty|title=(help) - Aust, E.F.; S. Ito; M. Sawodny; W. Knoll (1994). Trends in Polymer Science. 2: 313.
{{cite journal}}: Missing or empty|title=(help) - Tae, G.; J.A. Kornfield; J.A. Hubbell; Diethelm Johannsmann (17 September 2002). "Anomalous Sorption in Thin Films of Fluoroalkyl-Ended Poly(Ethylene Glycol)s". Langmuir. 18 (21): 8241–8245. doi:10.1021/la020255l.
- Yuan, Jipei; Dan Wen; Nikolai Gaponik; Alexander Eychmuller (22 November 2012). "Enzyme-Encapsulating Quantum Dot Hydrogels and Xerogels as Biosensors: Multifunctional Platforms for Both Biocatalysis and Fluorescent Probing". Angewandte Chemie International Edition. 52 (3): 976–979. doi:10.1002/anie.201205791. PMID 23172829.
- Roullier, Victor; Fabien Grasset; Fouzia Boulmedais; Franck Artzner; Olivier Cador; Vale´rie Marchi-Artzner (15 October 2008). "Small Bioactivated Magnetic Quantum Dot Micelles" (PDF). Chemistry of Materials. 20 (21): 6657–6665. doi:10.1021/cm801423r. Retrieved 8 June 2013.
- Huff, Terry; Ling Tong; Yan Zhao; Matthew Hansen; Jin-Xin Cheng; Alexander Wei (2007). "Hyperthermic effect of gold nanorods on tumor cells" (PDF). Nanomedicine. 2 (1): 125–132. doi:10.2217/17435889.2.1.125. PMC 2597406. PMID 17716198.