Hydrogenated MDI
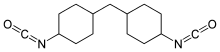
Hydrogenated MDI (H12MDI or 4,4′-diisocyanato dicyclohexylmethane) is an organic compound in the class known as isocyanates.[1] More specifically, it is an aliphatic diisocyanate. It is a water white liquid at room temperature and is manufactured in relatively small quantities. It is also known as 4,4'-methylenedi(cyclohexyl isocyanate) or methylene bis(4-cyclohexylisocyanate)[2] and has the formula CH2[(C6H10)NCO]2.
 | |
| Names | |
|---|---|
| IUPAC name
1-isocyanato-4-[(4-isocyanatocyclohexyl)methyl]cyclohexane | |
| Other names
H12MDI 4,4′-diisocyanato dicyclohexylmethane dicyclohexylmethane-4,4'-diisocyanate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.023.512 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 2206 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H22N2O2 | |
| Molar mass | 262.353 g·mol−1 |
| Hazards | |
| GHS labelling: | |
   | |
| Danger | |
| H315, H317, H319, H331, H334, H335 | |
| P261, P264, P271, P272, P280, P285, P302+P352, P304+P340, P304+P341, P305+P351+P338, P311, P312, P321, P332+P313, P333+P313, P337+P313, P342+P311, P362, P363, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Manufacture
The product is manufactured by hydrogenation of methylene diphenyl diisocyanate. It may also be manufactured by phosgenation of 4,4-Diaminodicyclohexylmethane.
Uses
Aliphatic diisocyanates are not used in the production of polyurethane foam as the cost is too high and foam is very much a commodity. It is used in special applications for polyurethane, such as enamel coatings which are resistant to abrasion and degradation from ultraviolet light. There are also multiple patents where prepolymers based on it are used in golf ball production.[3] It is available commercially under the tradename of Desmodur W from Covestro - formerly Bayer Material Science. It is used as a reactive building block for the preparation of other chemical products such as isocyanate terminated prepolymers and other urethane polymers.[4] The isocyanate groups can undergo addition reactions at room temperature with compounds which contain active hydrogens especially amines and polyols. Polyurethane resins based on this diisocyanate have good flexibility and mechanical strength. The polymers formed tend to have abrasion and hydrolysis resistance as well as retaining gloss and physical properties upon weathering. The resins based on this material are useful in coatings for flooring, roofing, maintenance and adhesives, and sealants. They find use in the coatings, adhesives, sealants and elastomers (CASE) applications.[5][6] A prepolymer made from H12MDI and incorporating dimethylol propionic acid can also be converted to light stable polyurethane dispersions.[7][8]
See also
References
- PubChem. "Bis(4-isocyanatocyclohexyl)methane". pubchem.ncbi.nlm.nih.gov. Retrieved 2021-01-18.
- "CDC - NIOSH Pocket Guide to Chemical Hazards - Methylene bis(4-cyclohexylisocyanate)". www.cdc.gov. Retrieved 2021-01-18.
- US 6593443, Iwami, Satoshi, "Wound-core golf ball", published 2003-07-15, issued 2001-09-05, assigned to Sumitomo Rubber Industries
- Howarth, GA (2003-06-01). "Polyurethanes, polyurethane dispersions and polyureas: Past, present and future". Surface Coatings International Part B: Coatings Transactions. 86 (2): 111–118. doi:10.1007/BF02699621. ISSN 1476-4865. S2CID 93574741.
- US 7598336, Fukuda, Keisuke; Nagahisa, Seiji & Yamaguchi, Hiroshi, "Two-part curing high-durable polyurethane elastomer composition", published 2009-10-06, issued 2004-12-10, assigned to Nihon Gosei Kako Co. Ltd.
- EP 3067377, Pela, Roberto; Kinzelmann, Hans-Georg & Wang, Yongxia et al., "Ultralow monomer polyurethanes", published 2016-09-14, assigned to Henkel AG & C. KGaA and Henkel IP & Holding GMBH
- Howarth, G. A.; Manock, H. L. (1997-07-01). "Water-borne polyurethane dispersions and their use in functional coatings". Surface Coatings International. 80 (7): 324–328. doi:10.1007/BF02692680. ISSN 1356-0751. S2CID 137433262.
- Chen, S. (2021). "Synthetic scheme to increase the abrasion resistance of waterborne polyurethane–urea by controlling micro-phase separation". J Appl Polym Sci. 138 (24): e50561. doi:10.1002/app.50561. ISSN 1097-4628. S2CID 233912583.