Hydroxyanthraquinone
A hydroxyanthraquinone (formula: C14H7O2(OH)) is any of several organic compounds that can be viewed as derivatives of an anthraquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH).
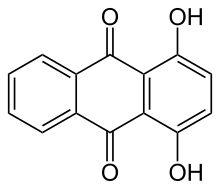
The IUPAC nomenclature recommends hydroxyanthracenedione.
Usually "hydroxyanthraquinone" refers to a derivative of 9,10-anthraquinone.[3][4][5]
Isomers
In general, the term may mean any anthraquinone derivative where any number n of hydrogens have been replaced by n hydroxyls, so that the formula is C
14H
10O
2+n. In this case, the number n (which is between 1 and 8) is indicated by a multiplier prefix (mono-, di-, tri-, up to octa-). Additional hydroxy- compounds can be derived from the other isomers of the latter. From 9,10-anthraquinone, only two single-hydroxy derivatives are possible.
See also
References
- Bien, H.-S.; Stawitz, J.; Wunderlich, K. "Anthraquinone Dyes and Intermediates". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_355.
- Bigelow, L. A.; Reynolds, H. H. (1926). "Quinizarin". Org. Synth. 6: 78. doi:10.15227/orgsyn.006.0078.
- Khalafy, J.; Bruce, J. M. (2002). "Oxidative Dehydrogenation of 1-Tetralones: Synthesis of Juglone, Naphthazarin, and α-Hydroxyanthraquinones" (pdf). Journal of Sciences, Islamic Republic of Iran. 13 (2): 131–139.
- Thomson, R. H. (1971). Naturally Occurring Quinones. London: Academic Press. Quoted by Khalafy and Bruce.
- Thomson, R. H. (1987). Naturally Occurring Quinones III. London: Chapman and Hall. Quoted by Khalafy and Bruce.