Hydroxycitric acid
Hydroxycitric acid (HCA) is a derivative of citric acid that is found in a variety of tropical plants including Garcinia cambogia and Hibiscus sabdariffa.[1]
 | |
| Names | |
|---|---|
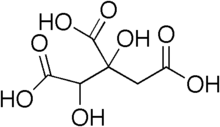
| Preferred IUPAC name
1,2-Dihydroxypropane-1,2,3-tricarboxylic acid | |
| Other names
Hydroxycitrate (anion name) | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C6H8O8 | |
| Molar mass | 208.122 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
There are four isomers, (+)- and (-)-hydroxycitric acid, and (+)- and (-)-allo-hydroxycitric acid. The (-)-hydroxycitric acid isomer is the one found in Garcinia.[2]
Chemistry
Hydroxy citric acid as such cannot be isolated from garcinia fruits or hibiscus sabdariffa fruits. Hydroxy citric acid exist in both the open and lactone forms. The presence of two chiral centres in the molecule is exploited to construct molecular skeletons that are otherwise difficult to synthesize, thus demonstrating the lactones use as chirons.[3]
Biological effects
(-)-HCA is a competitive inhibitor of ATP citrate lyase, which converts citrate into oxaloacetate and acetyl CoA.[2] The reverse of this conversion is a step in the citric acid cycle.
Laboratory and animal studies of HCA have produced results that indicate a potential for modulation of lipid metabolism.[4] However, a clinical study has demonstrated that HCA has no effect in terms of weight loss or reduction of fat mass.[5] A meta-analysis published in 2010 revealed that gastrointestinal adverse effects were twice as likely for users of hydroxycitric acid. The use of HCA is contraindicated in patients suffering Colitis or Inflammatory Bowel Disease.[6]
One isomer of HCA, known as (2S,3R)-HCA, inhibits pancreatic alpha-amylase and intestinal alpha-glucosidase, leading to a reduction in carbohydrate metabolism in vitro.[1] In a study in Zucker rats, which are genetically predisposed to obesity, Garcinia cambogia extract containing HCA showed that high doses led to significant suppression of epididymal fat accumulation, but also had high testicular toxicity.[7] However, this study has been criticized because of possible contamination of the HCA used and various design flaws.[8][9]
Researchers at the University of Houston reported hydroxycitrate is capable of dissolving calcium oxalate crystals, a component of human kidney stones. Recent studies (2019) shows kidney stones are layered and the stones may form and dissolve with time. The researchers believe the effect could lead to the development of new drugs for human kidney stones.[10]
References
- Yamada T, Hida H, Yamada Y (2007). "Chemistry, physiological properties, and microbial production of hydroxycitric acid". Appl. Microbiol. Biotechnol. 75 (5): 977–82. doi:10.1007/s00253-007-0962-4. PMID 17476502. S2CID 25194835.
- Jena, BS; Jayaprakasha, GK; Singh, RP; Sakariah, KK (2002-01-02). "Chemistry and biochemistry of (-)-hydroxycitric acid from Garcinia". Journal of Agricultural and Food Chemistry. 50 (1): 10–22. doi:10.1021/jf010753k. PMID 11754536.
- Habel, Deenamma; Nair, Divya S.; Kallingathodi, Zabeera; Mohan, Chithra; Pillai, Sarath M.; Nair, Rani R.; Thomas, Grace; Haleema, Simimole; Gopinath, Chithra; Abdul, Rinshad V.; Fritz, Matthew (2020-07-24). "Natural Product-Derived Chiral Pyrrolidine-2,5-diones, Their Molecular Structures and Conversion to Pharmacologically Important Skeletons". Journal of Natural Products. 83 (7): 2178–2190. doi:10.1021/acs.jnatprod.0c00211. ISSN 0163-3864. PMID 32584573. S2CID 220079442.
- Shara M, Ohia SE, Yasmin T, et al. (2003). "Dose- and time-dependent effects of a novel (−)-hydroxycitric acid extract on body weight, hepatic and testicular lipid peroxidation, DNA fragmentation and histopathological data over a period of 90 days". Mol. Cell. Biochem. 254 (1–2): 339–46. doi:10.1023/A:1027358106407. PMID 14674714. S2CID 25594704.
- Heymsfield SB, Allison DB, Vasselli JR, Pietrobelli A, Greenfield D, Nunez C (1998). "Garcinia cambogia (hydroxycitric acid) as a potential antiobesity agent: a randomized controlled trial". JAMA. 280 (18): 1596–600. doi:10.1001/jama.280.18.1596. PMID 9820262.
- Igho O, Shao K, Rachel P, Barbara W, Edzard E (2011). "The Use of Garcinia Extract Hydroxycitric Acid as a Weight loss Supplement: A Systematic Review and Meta-Analysis of Randomised Clinical Trials". J. Obes. 2011 (622): 1–9. doi:10.1155/2011/509038. PMC 3010674. PMID 21197150.
- Saito M, Ueno M, Ogino S, Kubo K, Nagata J, Takeuchi M (2005). "High dose of Garcinia cambogia is effective in suppressing fat accumulation in developing male Zucker obese rats, but highly toxic to the testis". Food Chem. Toxicol. 43 (3): 411–9. doi:10.1016/j.fct.2004.11.008. PMID 15680676.
- Madhusudan Soni Burdock Group (2005). "Garcinia cambogia toxicity is misleading". Food and Chemical Toxicology. 43 (11): 1683–1684. doi:10.1016/j.fct.2005.05.011. PMID 15993998.
- Hayamizu, K; Tomi, H; Kaneko, I; Shen, M; Soni, MG; Yoshino, G (2008). "Effects of Garcinia cambogia extract on serum sex hormones in overweight subjects". Fitoterapia. 79 (4): 255–61. doi:10.1016/j.fitote.2007.12.003. PMID 18316163.
- Chung J, Granja I, Taylor M, Mpourmpakis G, Asplin J (2016). "Molecular modifiers reveal a mechanism of pathological crystal growth inhibition". Nature. 536 (7617): 446–450. Bibcode:2016Natur.536..446C. doi:10.1038/nature19062. PMID 27501150. S2CID 4452431.