Hydroxylamine-O-sulfonic acid
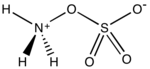
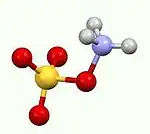
Hydroxylamine-O-sulfonic acid (HOSA) or aminosulfuric acid is the inorganic compound with molecular formula H3NO4S that is formed by the sulfonation of hydroxylamine with oleum.[1] It is a white, water-soluble and hygroscopic, solid, commonly represented by the condensed structural formula H2NOSO3H, though it actually exists as a zwitterion[2] and thus is more accurately represented as +H3NOSO3−. It is used as a reagent for the introduction of amine groups (–NH2), for the conversion of aldehydes into nitriles and alicyclic ketones into lactams (cyclic amides), and for the synthesis of variety of nitrogen-containing heterocycles.[2][3][4]
 | |
 | |
| Names | |
|---|---|
| Other names
Aminosulfuric acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.019.065 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| H3NO4S | |
| Molar mass | 113.09 |
| Appearance | white solid |
| Melting point | 210 °C |
| cold water | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Preparation
According to a laboratory procedure[1] hydroxylamine-O-sulfonic acid can be prepared by treating hydroxylamine sulfate with fuming sulfuric acid (oleum). The industrial process is similar.[5]
- (NH3OH)2SO4 + 2SO3 → 2H2NOSO3H + H2SO4
The sulfonation of hydroxylamine can also be effected with chlorosulfonic acid[2] by a method first published in 1925[6] and refined for Organic Syntheses.[7]
Structure
Analogous to sulfamic acid (H3N+SO3−) and as is the case generally for amino acids, HOSA exists in the solid state as a zwitterion: H3N+OSO3−. It resembles an ammonia molecule coordinate covalently bonded to a sulfate group.[8]
Reactions
HOSA reacts under basic conditions as nucleophile and under neutral and acid conditions as electrophile.[3][9]
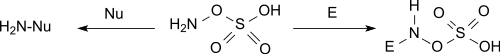
Aminations
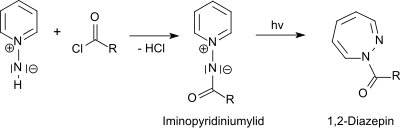
It reacts with tertiary amines to trisubstituted hydrazinium salts and with pyridine to the 1-amino pyridinium salt.[10]
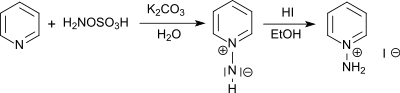
From 1-aminopyridinium salts the photochemically active 1-N-iminopyridinium ylides are accessible by acylation.[11] The photochemical rearrangement of the obtained 1-N-iminipyridinium ylides leads in high yields to 1H-1,2-diazepines[12]
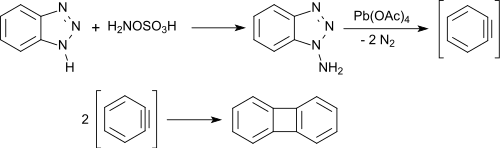
N-amination of 1H-benzotriazole with hydroxylamine-O-sulfonic acid yields a mixture of 1-aminobenzotriazole (major product) and 2-aminobenzotriazole (minor product). From 1-aminotriazole, benzyne is formed in an almost quantitative yield by oxidation with lead(IV) acetate, which rapidly dimerizes to biphenylene in good yields.[13]
Electron deficient heterocycles, such as tetrazole, can be N-aminated with hydroxylamine-O-sulfonic acid, while even more electron-deficient compounds, such as 5-nitrotetrazole, react only with stronger aminating agents such as O-tosylhydroxylamine or O- mesitylene sulfonylhydroxylamine to amino compounds, which were investigated as explosives.[14]
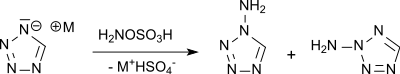
In the N-amination of the unsubstituted tetrazole, a mixture of 1-amino- and 2-aminotetrazole is obtained.
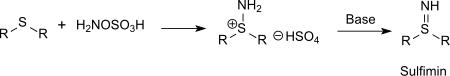
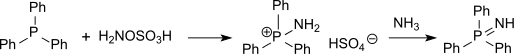
Also sulfur compounds (such as thioethers) can be aminated with hydroxylamine-O-sulfonic acid to sulfinimines (isosteric with sulfoxides but far more unstable) or phosphorus compounds (such as triphenylphosphine) can be aminated to phosphine imides via the intermediate aminotriphenylphosphonium hydrogen sulfate.[15]
The reaction of hydroxylamine-O-sulfonic acid with metal salts of sulfinic acids in sodium acetate solution produces primary sulfonamides in very good yields.[16]
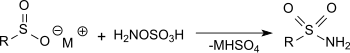
Diimine can formed in situ from hydroxylamine-O-sulfonic acid respectively hydroxylamine-O-sulfonic acid hydroxylamine sulfate mixtures, which hydrogenates selectively conjugated multiple bonds.[20]
With carbonyl compounds
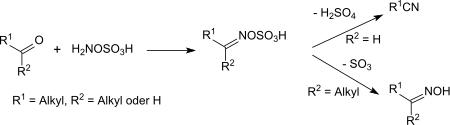
At room temperature and below, hydroxylamine-O-sulfonic acid reacts with ketones and aldehydes as a nucleophile to the corresponding oxime-O-sulfonic acids or their salts.[17] The oxime-O-sulfonic acids of aldehydes react above room temperature upon elimination of sulfuric acid in high yields to nitriles.[18]
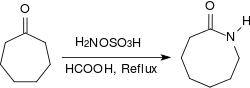
Aliphatic ketones provide under similar conditions in very high yields oximes, arylalkyl ketones react in a Beckmann rearrangement to amides. When heated to reflux for several hours under acidic conditions (e.g., in the presence of concentrated formic acid) alicyclic ketones react to provide lactams in high yields.[19]
Under basic conditions in the presence of primary amines, hydroxylamine-O-sulfonic acid forms with aldehydes and ketones (e.g. cyclohexanone[20]) diaziridines, which can easily be oxidized to the more stable diazirines.
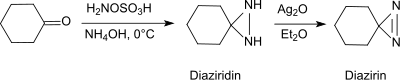
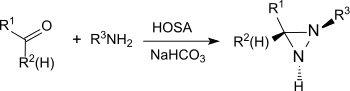
The reaction also provides substituted aziridines from simple aldehydes and ketones with high yield and diastereoselectivity.[21]
1,2-Benzisoxazole is efficiently produced by nucleophilic attack of hydroxylamine-O-sulfonic acid to the carbonyl group of 2-hydroxybenzaldehyde followed by cyclization.[22]
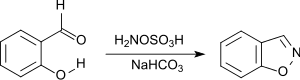
1,2-Benzisoxazole is a structural element in the antipsychotic risperidone and paliperidone, as well as the anticonvulsant zonisamide.
In a one-pot reaction, N-aryl[3,4-d]pyrazolopyrimidines are obtained in good yields from simple 4,6-dichloropyrimidine-5-carboxaldehyde,[23]
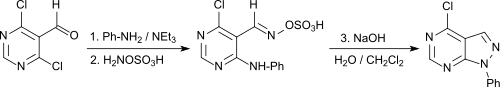
which can be used as purine analogs for a wide range of diagnostic and therapeutic applications.[24]
Further reactions
The chemiluminescence of the system luminol/cobalt(II) chloride is dramatically enhanced by the addition of hydroxylamine-O-sulfonic acid.[25]
References
- Matsuguma, Harold J.; Audrieth, Ludwig F.; Wehrmeister, Herbert L. (1957). "Hydroxylamine‐ O ‐Sulfonic Acid". Inorganic Syntheses. pp. 122–125. doi:10.1002/9780470132364.ch32. ISBN 9780470132364.
{{cite book}}:|journal=ignored (help) - Wiberg, Egon; Wiberg, Nils (2001). "Sulfur Compounds of Nitrogen". Inorganic Chemistry. Academic Press. pp. 675–677. ISBN 978-0-12-352651-9.
- Wallace, Raymond G. (1980). "Hydroxylamine-O-sulfonic acid – a versatile synthetic reagent". Aldrichimica Acta. 13 (1): 3–11.
- Rademacher, P. (2014). "Product Class 7: Hydrazines and Hydrazinium Salts (40.7.1.1.9.2 – Using Hydroxylamine-O-sulfonic Acids". In Enders, Dieter; Schaumann, E. (eds.). Compounds with One Saturated Carbon–Heteroatom Bond: Amine N-Oxides, Haloamines, Hydroxylamines and Sulfur Analogues, and Hydrazines. Science of Synthesis: Houben-Weyl Methods of Molecular Transformations. Vol. 40b. Georg Thieme Verlag. p. 1171. ISBN 978-3-13-172181-5.
- US patent 3281209, Wehrmeister, Herbert L. & Yalowitz, Harold I., "Process for the preparation of hydroxylamine-O-sulfonic acid", published 1966-10-25, issued 1966-10-25, assigned to Commercial Solvents Corporation
- Sommer, F.; Schulz, O. F.; Nassau, M. (1925). "Über die Sulfoperamidsäure" [About Sulfoperamic Acid]. Z. Anorg. Allg. Chem. (in German). 147 (1): 142–155. doi:10.1002/zaac.19251470115.
- Rathke, Michael W.; Millard, Alan A. (1978). "Boranes in functionalization of olefins to amines: 3-Pinanamine (Bicyclo[3.1.1]heptan-3-amine, 2,6,6-trimethyl-)". Organic Syntheses. 58: 32. doi:10.15227/orgsyn.058.0032.; Collective Volume, vol. 6, p. 943
- Baenziger, Norman C.; Belt, Roger F.; Goebel, Carol V. (1967). "Crystal structure of hydroxylamine-O-sulfonic acid". Inorg. Chem. 6 (3): 511–514. doi:10.1021/ic50049a017.
- Erdik, Ender (2001). "Hydroxylamine-O-sulfonic Acid". Hydroxylamine-O-Sulfonic Acid. Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rh058. ISBN 978-0-471-93623-7.
- R. Gösl; A. Meuwsen (1963). "1-Aminopyridinium iodide". Org. Synth. (in German). 43: 1. doi:10.15227/orgsyn.043.0001.
- J. Streith (1991). "The Photochemistry of N-Iminopyridinium Ylides in Retrospect. From a Simple Concept to Some Applications". CHIMIA (in German). 45 (3): 65–76.
- J. Streith (1977). "The photochemistry of aromatic-N-ylides. Rearrangement and fragmentation patterns". Pure Appl. Chem. (in German). 49 (3): 305–315. doi:10.1351/pac197749030305.
- Campbell, C.D.; Rees, C.W. (1969). "Reactive intermediates. Part I. Synthesis and oxidation of 1- and 2-aminobenzotriazole". J. Chem. Soc. C. 1969 (5): 742–747. doi:10.1039/J39690000742.
- T.M. Klapötke; D.G. Piercey; J. Stierstorfer (2012). "Amination of energetic anions: high-performing energetic materials". Dalton Trans. (in German). 41 (31): 9451–9459. doi:10.1039/C2DT30684K. PMID 22751656.
- R. Appel; W. Büchner; E. Guth (1958). "Zur Kenntnis des Imins, I. Über Phosphinimine und Sulfinimine". Justus Liebigs Ann. Chem. (in German). 618 (1): 53–58. doi:10.1002/jlac.19586180107.
- S.L. Graham; T.H. Scholz (1986). "The reaction of sulfinic acid salts with hydroxylamine-O-sulfonic acid. A useful synthesis of primary sulfonamides". Synthesis (in German). 1986 (2): 1031–1032. doi:10.1055/s-1986-31862.
- J. Streith; C. Fizet (1977). "Nucleophilic versus electrophilic properties of the nitrogen atom in O-sulfonyl-hydroxylamine derivatives". Tetrahedron Lett. (in German). 18 (37): 3297–3300. doi:10.1016/S0040-4039(01)83223-8.
- C. Fizet; J. Streith (1974). "Hydroxylamine-O-sulfonic acid: A convenient reagent for the oxidative conversion of aldehydes into nitriles". Tetrahedron Lett. (in German). 15 (36): 3187–3188. doi:10.1016/S0040-4039(01)91857-X.
- G.A. Olah; A.P. Fung (1985). "Hexahydro-2-(1H)-azocinone". Org. Synth. (in German). 63: 188. doi:10.15227/orgsyn.063.0188.
- E. Schmitz; R. Ohme (1965). "3,3-Pentamethylenediaziridine". Org. Synth. (in German). 45: 83. doi:10.15227/orgsyn.045.0083.
- A.W. Beebe; E.F. Dohmeier; G. Moura-Letts (2015). "Diastereoselective synthesis of substituted diaziridines from simple ketones and aldehydes". Chem. Commun. (in German). 51 (70): 13511–13514. doi:10.1039/C5CC04813C. PMID 26216745.
- D.S. Kemp; R.B. Woodward (1965). "The N-ethylbenzisoxazolium cation—I : Preparation and reactions with nucleophilic species". Tetrahedron (in German). 21 (11): 3019–3035. doi:10.1016/S0040-4020(01)96921-2.
- L.E. Evans; M.D. Cheeseman; K. Jones (2012). "N–N Bond-Forming Cyclization for the One-Pot Synthesis of N-Aryl[3,4-d]pyrazolopyrimidines". Org. Lett. (in German). 14 (13): 3546–3549. doi:10.1021/ol301561a. PMC 3390909. PMID 22734502.
- C. Morrill; S. Babu; N.G. Almstead; Y.-C. Moon (2013). "Synthesis of 1,4-disubstituted pyrazolo[3,4-d]pyrimidines from 4,6-dichloropyrimidine-5-carboxaldehyde: insights into selectivity and reactivity". Synthesis (in German). 45 (13): 1791–1806. doi:10.1055/s-0033-1338862.
- M. Saqib; W. Gao; J. Lai; L. Qi; S. Majeed; M.R.H.S. Gilani; G. Xu (2015). "Hydroxylamine-O-sulfonic acid as an efficient coreactant for luminol chemiluminescence for selective and sensitive detection". Chem. Commun. (in German). 51 (30): 6536–6539. doi:10.1039/C5CC01090J. PMID 25766485.