Vitamin D deficiency
Vitamin D deficiency or hypovitaminosis D is a vitamin D level that is below normal. It most commonly occurs in people when they have inadequate exposure to sunlight, particularly sunlight with adequate ultraviolet B rays (UVB).[1][2][3] Vitamin D deficiency can also be caused by inadequate nutritional intake of vitamin D; disorders that limit vitamin D absorption; and disorders that impair the conversion of vitamin D to active metabolites, including certain liver, kidney, and hereditary disorders.[4] Deficiency impairs bone mineralization, leading to bone-softening diseases, such as rickets in children. It can also worsen osteomalacia and osteoporosis in adults, increasing the risk of bone fractures.[1][4] Muscle weakness is also a common symptom of vitamin D deficiency, further increasing the risk of fall and bone fractures in adults.[1] Vitamin D deficiency is associated with the development of schizophrenia.[5]
| Vitamin D deficiency | |
|---|---|
| Other names | Hypovitaminosis D |
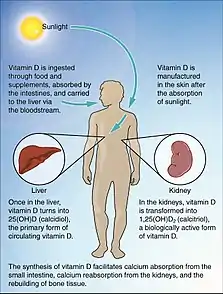 | |
| The normal process of Vitamin D absorption | |
| Specialty | Endocrinology |
| Symptoms | Usually asymptomatic |
| Complications | Rickets, osteomalacia, other associated disorders |
| Causes | Lack of vitamin D, inadequate sunlight exposure |
| Risk factors | Age, people with dark skin, obesity, malabsorption, bariatric surgery, breastfed infants[1] |
| Diagnostic method | Measuring the concentration of calcifediol in the blood |
| Prevention | Sufficient sunlight exposure, dietary intake |
| Treatment | Supplements |
| Medication | Cholecalciferol, ergocalciferol, calcifediol |
| Frequency | Severe deficiency (<30 nmol/L): Europe 13%, US 5.9%, Canada 7.4%. Deficiency (<50 nmol/L): Europe 40%, US 24%, Canada 37%[2] |
Vitamin D can be synthesized in the skin under the exposure of UVB from sunlight. Oily fish, such as salmon, herring, and mackerel, are also sources of vitamin D, as are mushrooms. Milk is often fortified with vitamin D; sometimes bread, juices, and other dairy products are fortified with vitamin D.[1] Many multivitamins contain vitamin D in different amounts.[1]
Classifications


Vitamin D deficiency is typically diagnosed by measuring the concentration of the 25-hydroxyvitamin D in the blood, which is the most accurate measure of stores of vitamin D in the body.[1][7][2] One nanogram per millilitre (1 ng/mL) is equivalent to 2.5 nanomoles per litre (2.5 nmol/L).
- Severe deficiency: <12 ng/mL = <30 nmol/L[2]
- Deficiency: <20 ng/mL = <50 nmol/L
- Insufficient: 20–29 ng/mL = 50–75 nmol/L
- Normal: 30–50 ng/mL = 75–125 nmol/L
Vitamin D levels falling within this normal range prevent clinical manifestations of vitamin D insufficiency as well as vitamin D toxicity.[1][7][2]
Signs and symptoms

In most cases, vitamin D deficiency is almost asymptomatic.[8] It may only be detected on blood tests but is the cause of some bone diseases and is associated with other conditions:[1]
Complications
- Rickets, a childhood disease characterized by impeded growth and deformity of the long bones.[9] The earliest sign of vitamin D deficiency is craniotabes, abnormal softening or thinning of the skull.[10]
- Osteomalacia, a bone-thinning disorder that occurs exclusively in adults and is characterized by proximal muscle weakness and bone fragility. Women with vitamin D deficiency who have been through multiple pregnancies are at elevated risk of osteomalacia.[11]
- Osteoporosis, a condition characterized by reduced bone mineral density and increased bone fragility
- Increased risk of fracture[12][13]
- Myopathy: Muscle aches, weakness, and twitching (fasciculations), due to reduced blood calcium (hypocalcemia),[3][14] impaired muscle glycogen metabolism (abnormal glycogen accumulation), atrophy of type II (fast-twitch/glycolytic) muscle fibres, and diminished calcium uptake by the sarcoplasmic reticulum (needed for muscle contraction).[15]
- Periodontitis, local inflammatory bone loss that can result in tooth loss.[16]
- Pre-eclampsia: There has been an association of vitamin D deficiency and women who develop pre-eclampsia in pregnancy. The exact relationship of these conditions is not well understood.[17] Maternal vitamin D deficiency may affect the baby, causing overt bone disease from before birth and impairment of bone quality after birth.[9][18]
- Respiratory infections and COVID-19: Vitamin D deficiency may increase the risk of severe acute respiratory infections and COPD.[19][20] Emerging studies have suggested a link between vitamin D deficiency and COVID-19 symptoms.[21][22] A review has shown that vitamin D deficiency is not associated with a higher chance of having COVID-19 but is associated with a greater severity of the disease, including 80% increases in the rates of hospitalization and mortality.[23]
- Schizophrenia: Vitamin D deficiency is associated with the development of schizophrenia.[5] People with schizophrenia generally have lower levels of vitamin D.[24] The environmental risk factors of seasonality of birth, latitude, and migration linked to schizophrenia all implicate vitamin D deficiency, as do other health conditions such as maternal obesity.[5][25] Vitamin D is essential for the normal development of the nervous system.[5][24] Maternal vitamin D deficiency can cause prenatal neurodevelopmental defects, which influence neurotransmission, altering brain rhythms and the metabolism of dopamine.[24] Vitamin D receptors, CYP27B1, and CYP24A1 are found in various regions of the brain, showing that vitamin D is a neuroactive, neurosteroid hormone essential for the development of the brain and normal function.[5] Inflammation as a causative factor in schizophrenia is normally suppressed by vitamin D.[24]
Risk factors
Those most likely to be affected by vitamin D deficiency are people with little exposure to sunlight.[26] Certain climates, dress habits, the avoidance of sun exposure and the use of too much sunscreen protection can all limit the production of vitamin D.[26]
Age
Elderly people have a higher risk of having a vitamin D deficiency due to a combination of several risk factors, including decreased sunlight exposure, decreased intake of vitamin D in the diet, and decreased skin thickness, which leads to further decreased absorption of vitamin D from sunlight.[27]
Fat percentage
Since vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol) are fat-soluble, humans and other animals with a skeleton need to store some fat. Without fat, the animal will have a hard time absorbing vitamin D2 and vitamin D3, and the lower the fat percentage, the greater the risk of vitamin deficiency, which is true in some athletes who strive to get as lean as possible.[28]
Malnutrition
Although rickets and osteomalacia are now rare in Britain, osteomalacia outbreaks in some immigrant communities included women with seemingly adequate daylight outdoor exposure wearing typical Western clothing.[29] Having darker skin and reduced exposure to sunshine did not produce rickets unless the diet deviated from a Western omnivore pattern characterized by high intakes of meat, fish, and eggs and low intakes of high-extraction cereals.[30][31][32] In sunny countries where rickets occurs among older toddlers and children, the rickets has been attributed to low dietary calcium intakes. This is characteristic of cereal-based diets with limited access to dairy products.[32] Rickets was formerly a major public health problem among the US population; in Denver, almost two-thirds of 500 children had mild rickets in the late 1920s.[33] An increase in the proportion of animal protein in the 20th-century American diet coupled with increased consumption of milk fortified with relatively small quantities of vitamin D coincided with a dramatic decline in the number of rickets cases.[34][35][36] One study of children in a hospital in Uganda, however, showed no significant difference in vitamin D levels of malnourished children compared to non-malnourished children. Because both groups were at risk due to darker skin pigmentation, both groups had vitamin D deficiency. Nutritional status did not appear to play a role in this study.[37]
Obesity
There is an increased risk of vitamin D deficiency in people who are considered overweight or obese based on their body mass index (BMI) measurement.[38] The relationship between these conditions is not well understood. There are different factors that could contribute to this relationship, particularly diet and sunlight exposure.[38] Alternatively, vitamin D is fat-soluble, so excess amounts can be stored in fat tissue and used during winter, when sun exposure is limited.[39]
Sun exposure
The use of sunscreen with a sun protection factor of 8 can theoretically inhibit more than 95% of vitamin D production in the skin.[34] In practice, however, sunscreen is applied so as to have a negligible effect on vitamin D status.[40] The vitamin D status of those in Australia and New Zealand is unlikely to have been affected by campaigns advocating sunscreen.[41] Instead, wearing clothing is more effective at reducing the amount of skin exposed to UVB and reducing natural vitamin D synthesis. Clothing that covers a large portion of the skin, when worn on a consistent and regular basis, such as the burqa, is correlated with lower vitamin D levels and an increased prevalence of vitamin D deficiency.[42]
Regions far from the equator have a high seasonal variation of the amount and intensity of sunlight. In the UK, the prevalence of low vitamin D status in children and adolescents is found to be higher in winter than in summer.[43] Lifestyle factors such as indoor versus outdoor work and time spent in outdoor recreation play an important role.
Additionally, vitamin D deficiency has been associated with urbanisation in terms of both air pollution, which blocks UV light, and an increase in the number of people working indoors. The elderly are generally exposed to less UV light due to hospitalisation, immobility, institutionalisation, and being housebound, leading to decreased levels of vitamin D.[44]
Darker skin color
Because of melanin which enables natural sun protection, dark-skinned people are susceptible to vitamin D deficiency.[6][45] Three to five times greater sun exposure is necessary for naturally darker skinned people to produce the same amount of vitamin D as those with light skin.[45]
Malabsorption
Rates of vitamin D deficiency are higher among people with untreated celiac disease,[46][47] inflammatory bowel disease, exocrine pancreatic insufficiency from cystic fibrosis, and short bowel syndrome,[47] which can all produce problems of malabsorption. Vitamin D deficiency is also more common after surgical procedures that reduce absorption from the intestine, including weight loss procedures.[48]
Critical illness
Vitamin D deficiency is associated with increased mortality in critical illness.[49] People who take vitamin D supplements before being admitted for intensive care are less likely to die than those who do not take vitamin D supplements.[49] Additionally, vitamin D levels decline during stays in intensive care.[50] Vitamin D3 (cholecalciferol) or calcitriol given orally may reduce the mortality rate without significant adverse effects.[50]
Breastfeeding
Infants who exclusively breastfeed need a vitamin D supplement, especially if they have dark skin or have minimal sun exposure.[51] The American Academy of Pediatrics recommends that all breastfed infants receive 400 international units (IU) per day of oral vitamin D.[51]
Pathophysiology
Decreased exposure of the skin to sunlight is a common cause of vitamin D deficiency.[1] People with a darker skin pigment with increased amounts of melanin may have decreased production of vitamin D.[3] Melanin absorbs ultraviolet B radiation from the sun and reduces vitamin D production.[3] Sunscreen can also reduce vitamin D production.[3] Medications may speed up the metabolism of vitamin D, causing a deficiency.[3]
The liver is required to transform vitamin D into 25-hydroxyvitamin D. This is an inactive metabolite of vitamin D but is a necessary precursor (building block) to create the active form of vitamin D.[1]
The kidneys are responsible for converting 25-hydroxyvitamin D to 1,25-hydroxyvitamin D. This is the active form of vitamin D in the body. Kidney disease reduces 1,25-hydroxyvitamin D formation, leading to a deficiency of the effects of vitamin D.[1]
Intestinal conditions that result in malabsorption of nutrients may also contribute to vitamin D deficiency by decreasing the amount of vitamin D absorbed via diet.[1] In addition, a vitamin D deficiency may lead to decreased absorption of calcium by the intestines, resulting in increased production of osteoclasts that may break down a person's bone matrix.[52] In states of hypocalcemia, calcium will leave the bones and may give rise to secondary hyperparathyroidism, which is a response by the body to increase serum calcium levels.[52] The body does this by increasing uptake of calcium by the kidneys and continuing to take calcium away from the bones.[52] If prolonged, this may lead to osteoporosis in adults and rickets in children.[52]
Diagnosis
The serum concentration of calcifediol, also called 25-hydroxyvitamin D (abbreviated 25(OH)D), is typically used to determine vitamin D status. Most vitamin D is converted to 25(OH)D in the serum, giving an accurate picture of vitamin D status.[53] The level of serum 1,25(OH)D is not usually used to determine vitamin D status because it often is regulated by other hormones in the body such as parathyroid hormone.[53] The levels of 1,25(OH)D can remain normal even when a person may be vitamin D deficient.[53] Serum level of 25(OH)D is the laboratory test ordered to indicate whether or not a person has vitamin D deficiency or insufficiency.[53] It is also considered reasonable to treat at-risk persons with vitamin D supplementation without checking the level of 25(OH)D in the serum, as vitamin D toxicity has only been rarely reported to occur.[53]
Levels of 25(OH)D that are consistently above 200 nanograms per milliliter (ng/mL) (or 500 nanomoles per liter, nmol/L) are potentially toxic.[54] Vitamin D toxicity usually results from taking supplements in excess.[55] Hypercalcemia is often the cause of symptoms,[55] and levels of 25(OH)D above 150 ng/mL (375 nmol/L) are usually found, although in some cases 25(OH)D levels may appear to be normal. Periodic measurement of serum calcium in individuals receiving large doses of vitamin D is recommended.[4]
Screening
The official recommendation from the United States Preventive Services Task Force is that for persons that do not fall within an at-risk population and are asymptomatic, there is not enough evidence to prove that there is any benefit in screening for vitamin D deficiency.[56]
Treatment
UVB exposure
Vitamin-D overdose is impossible from UV exposure: the skin reaches an equilibrium where the vitamin degrades as fast as it is created.[57][58]
Sun tanning
Light therapy
Exposure to photons (light) at specific wavelengths of narrowband UVB enables the body to produce vitamin D to treat vitamin D deficiency.[59]
Supplement
In the United States and Canada as of 2016, the amount of vitamin D recommended is 400 IU per day for children, 600 IU per day for adults, and 800 IU per day for people over age 70.[60][61] The Canadian Paediatric Society recommends that pregnant or breastfeeding women consider taking 2000 IU/day, that all babies who are exclusively breastfed receive a supplement of 400 IU/day, and that babies living north of 55°N get 800 IU/day from October to April.[62]
Treating vitamin D deficiency depends on the severity of the deficit.[63] Treatment involves an initial high-dosage treatment phase until the required serum levels are reached, followed by the maintenance of the acquired levels. The lower the 25(OH)D serum concentration is before treatment, the higher is the dosage that is needed in order to quickly reach an acceptable serum level.[63]
The initial high-dosage treatment can be given on a daily or weekly basis or can be given in form of one or several single doses (also known as stoss therapy, from the German word Stoß, meaning "push").[64]
Therapy prescriptions vary, and there is no consensus yet on how best to arrive at an optimum serum level. While there is evidence that vitamin D3 raises 25(OH)D blood levels more effectively than vitamin D2,[65] other evidence indicates that D2 and D3 are equal for maintaining 25(OH)D status.[63]
Daily or weekly or monthly dose
For treating rickets, the American Academy of Pediatrics (AAP) has recommended that pediatric patients receive an initial two to three months of treatment with "high-dose" vitamin D therapy. In this regime, the daily dose of cholecalciferol is 1,000 IU for newborns, 1,000 to 5,000 IU for 1- to 12-month-old infants, and 5,000 IU for patients over 1 year of age.[64]
For adults, other dosages have been called for. A review of 2008/2009 recommended dosages of 1,000 IU cholecalciferol per 10 ng/mL required serum increase, to be given daily over two to three months.[66] In another proposed cholecalciferol loading dose guideline for vitamin D-deficient adults, a weekly dosage is given, up to a total amount that is proportional to the required serum increase (up to the level of 75 nmL/L) and within certain body weight limits, to body weight.[67]
According to new data and practices relevant to vitamin D levels in the general population in France, to establish optimal vitamin D status and frequency of intermittent supplement dosing,[68] patients with or at high risk for osteoporosis and vitamin D deficiency should start supplementation with a loading phase consisting of 50,000 IU weekly of vitamin D for 8 weeks in patients with levels <20 ng/mL and 50,000 IU weekly for 4 weeks in patients with levels between 20 and 30 ng/mL. Subsequently, long-term supplementation should be prescribed as 50,000 IU monthly. Should pharmaceutical forms suitable for daily supplementation become available, patients displaying good treatment adherence could take a daily dose determined based on the 25(OH)D level.
Until now, there are no consistent data suggesting the ideal regimen of supplementation with vitamin D, and the question of the ideal time between doses is still of debate. Ish-Shalom et al.[69] performed a study in elderly women to compare the efficacy and safety of a daily dose of 1,500 IU to a weekly dose of 10,500 IU and to a dose of 45,000 IU given every 28 days for two months. They concluded that supplementation with vitamin D can be equally achieved with daily, weekly, or monthly dosing frequencies. Another study comparing daily, weekly, and monthly supplementation of vitamin D in deficient patients was published by Takacs et al.[70] They reported equal efficacy of 1,000 IU taken daily, 7,000 IU taken weekly, and 30,000 IU taken monthly. Nevertheless, these consistent findings differ from the report by Chel et al.,[71] in which a daily dose was more effective than a monthly dose. In that study, the compliance calculation could be questionable since only random samples of the returned medications were counted. In a study by De Niet et al.,[72] 60 subjects with vitamin D deficiency were randomized to receive 2,000 IU vitamin D3 daily or 50,000 IU monthly. They reported a similar efficacy of the two dosing frequencies, with the monthly dose providing more rapid normalization of vitamin D levels.
Single-dose therapy
Alternatively, a single-dose therapy is used for instance if there are concerns regarding the patient's compliance. The single-dose therapy can be given as an injection but is normally given in form of oral medication.[64]
Vitamin D doses and meals
The presence of a meal and the fat content of that meal may also be important. Because vitamin D is fat-soluble, it is hypothesized that absorption would be improved if patients are instructed to take their supplement with a meal. Raimundo et al.[73][74] performed different studies confirming that a high-fat meal increased the absorption of vitamin D3 as measured by serum 25(OH) D. A clinical report indicated that serum 25(OH) D levels increased by an average of 57% over a 2-month to 3-month period in 17 clinic patients after they were instructed to take their usual dose of vitamin D with the largest meal of the day.[75]
Another study conducted in 152 healthy men and women concluded that diets rich in monounsaturated fatty acids may improve and those rich in polyunsaturated fatty acids may reduce the effectiveness of vitamin D3 supplements.[76] In another study performed by Cavalier E. et al.,[77] 88 subjects received orally a single dose of 50,000 IU of vitamin D3 solubilized in an oily solution as two ampoules each containing 25,000 IU (D‐CURE®, Laboratories SMB SA, Brussels, Belgium) with or without a standardized high‐fat breakfast. No significant difference between fasting vs. fed conditions was observed.
Maintenance phase
Once the desired serum level has been achieved, be it by a high daily or weekly or monthly dose or by a single-dose therapy, the AAP recommendation calls for a maintenance supplementation of 400 IU for all age groups, with this dosage being doubled for premature infants, dark-skinned infants and children, children who reside in areas of limited sun exposure (>37.5° latitude), obese patients, and those on certain medications.[64]
Special cases
To maintain blood levels of calcium, therapeutic vitamin D doses are sometimes administered (up to 100,000 IU or 2.5 mg daily) to patients who have had their parathyroid glands removed (most commonly kidney dialysis patients who have had tertiary hyperparathyroidism, but also to patients with primary hyperparathyroidism) or with hypoparathyroidism.[78] Patients with chronic liver disease or intestinal malabsorption disorders may also require larger doses of vitamin D (up to 40,000 IU or 1 mg (1000 micrograms) daily).
Co-supplementation with vitamin K
The combination of vitamin D and vitamin K supplements has been shown in trials to improve bone quality.[79] As high intake of vitamin D is a cause of raised calcium levels (hypercalcemia), the addition of vitamin K may be beneficial in helping to prevent vascular calcification, particularly in people with chronic kidney disease.[80][81]
Bioavailability
Not all D3 deficiencies can be effectively supplemented or treated with vitamin D3 on its own. Older people or those who have fatty liver or metabolic syndrome have a reduced ability to absorb vitamin D3.[82] In addition, in overweight or obese persons, excessive adipose tissue can sequester D3 from the circulation and reduce its access to other tissues.[82] With age or in obesity, metabolic activation of D3 may be reduced by liver steatosis or by microbiome imbalance.[82][83] Since Vitamin D is fat-soluble, it's advised to be taken with a meal high in fat since it significantly increase its uptake in healthy individuals.[84]
For vitamin D3 to perform its hormonal roles, it is converted into its biologically active metabolite, calcifediol, also known as 25-hydroxyvitamin D3, an activation occurring by a hydroxylation reaction in the liver via the cytochrome P450 system, and in the gut microbiome.[85]
Epidemiology
The estimated percentage of the population with a vitamin D deficiency varies based on the threshold used to define a deficiency.
| Percentage of US population | Definition of insufficiency | Study | Reference |
|---|---|---|---|
| 69.5% | 25(OH)D less than 30 ng/mL | Chowdury et al. 2014 | [86] |
| 77% | 25(OH)D less than 30 ng/mL | Ginde et al. 2009 | [87] |
| 36% | 25(OH)D less than 20 ng/mL | Ginde et al. 2009 | [87] |
| 6% | 25(OH)D less than 10 ng/mL | Ginde et al. 2009 | [87] |
Recommendations for 25(OH)D serum levels vary across authorities, and probably vary based on factors like age; calculations for the epidemiology of vitamin D deficiency depend on the recommended level used.[88]
A 2011 Institute of Medicine (IOM) report set the sufficiency level at 20 ng/mL (50 nmol/L), while in the same year The Endocrine Society defined sufficient serum levels at 30 ng/mL and others have set the level as high as 60 ng/mL.[89] As of 2011 most reference labs used the 30 ng/mL standard.[63][89][90]: 435
Applying the IOM standard to NHANES data on serum levels, for the period from 1988 to 1994 22% of the US population was deficient, and 36% were deficient for the period between 2001 and 2004; applying the Endocrine Society standard, 55% of the US population was deficient between 1988 and 1994, and 77% were deficient for the period between 2001 and 2004.[89]
In 2011 the Centers for Disease Control and Prevention applied the IOM standard to NHANES data on serum levels collected between 2001 and 2006, and determined that 32% of Americans were deficient during that period (8% at risk of deficiency, and 24% at risk of inadequacy).[89][91]
History
The role of diet in the development of rickets was determined by Edward Mellanby between 1918 and 1920.[92] In 1921, Elmer McCollum identified an antirachitic substance found in certain fats that could prevent rickets. Because the newly discovered substance was the fourth vitamin identified, it was called vitamin D.[92] The 1928 Nobel Prize in Chemistry was awarded to Adolf Windaus, who discovered the steroid 7-dehydrocholesterol, the precursor of vitamin D.
Prior to the fortification of milk products with vitamin D, rickets was a major public health problem. In the United States, milk has been fortified with 10 micrograms (400 IU) of vitamin D per quart since the 1930s, leading to a dramatic decline in the number of rickets cases.[34]
Research
Some evidence suggests vitamin D deficiency may be associated with a worse outcome for some cancers, but evidence is insufficient to recommend that vitamin D be prescribed for people with cancer.[93] Taking vitamin D supplements has no significant effect on cancer risk.[94] Vitamin D3, however, appears to decrease the risk of death from cancer but concerns with the quality of the data exist.[95]
Vitamin D deficiency is thought to play a role in the pathogenesis of non-alcoholic fatty liver disease.[96][97]
Evidence suggests that vitamin D deficiency may be associated with impaired immune function.[98][99] Those with vitamin D deficiency may have trouble fighting off certain types of infections. It has also been thought to correlate with cardiovascular disease, type 1 diabetes, type 2 diabetes, and some cancers.[7]
Review studies have also seen associations between vitamin D deficiency and pre-eclampsia.[100]
References
- "Office of Dietary Supplements - Vitamin D". ods.od.nih.gov. Retrieved 31 October 2020.
- Amrein, K; Scherkl, M; Hoffmann, M; Neuwersch-Sommeregger, S; Köstenberger, M; Tmava Berisha, A; Martucci, G; Pilz, S; Malle, O (20 January 2020). "Vitamin D deficiency 2.0: an update on the current status worldwide". European Journal of Clinical Nutrition. 74 (11): 1498–1513. doi:10.1038/s41430-020-0558-y. PMC 7091696. PMID 31959942.
- Holick MF, Chen TC (April 2008). "Vitamin D deficiency: a worldwide problem with health consequences". The American Journal of Clinical Nutrition. 87 (4): 1080S–6S. doi:10.1093/ajcn/87.4.1080S. PMID 18400738.
- Vitamin D at Merck Manual of Diagnosis and Therapy Professional Edition
- Chiang M, Natarajan R, Fan X (February 2016). "Vitamin D in schizophrenia: a clinical review". Evidence-Based Mental Health. 19 (1): 6–9. doi:10.1136/eb-2015-102117. PMID 26767392. S2CID 206926835.
- Heaney RP (December 2004). "Functional indices of vitamin D status and ramifications of vitamin D deficiency". The American Journal of Clinical Nutrition. 80 (6 Suppl): 1706S–9S. doi:10.1093/ajcn/80.6.1706S. PMID 15585791.
- GR, Gupta A (February 2014). "Vitamin D deficiency in India: prevalence, causalities and interventions". Nutrients. 6 (2): 729–75. doi:10.3390/nu6020729. PMC 3942730. PMID 24566435.
- Sizar, Omeed; Khare, Swapnil; Goyal, Amandeep; Bansal, Pankaj; Givler, Amy (2021), "Vitamin D Deficiency", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 30335299, retrieved 2021-11-24
- Elidrissy AT (September 2016). "The Return of Congenital Rickets, Are We Missing Occult Cases?". Calcified Tissue International (Review). 99 (3): 227–36. doi:10.1007/s00223-016-0146-2. PMID 27245342. S2CID 14727399.
- Yorifuji J, Yorifuji T, Tachibana K, Nagai S, Kawai M, Momoi T, Nagasaka H, Hatayama H, Nakahata T (May 2008). "Craniotabes in normal newborns: the earliest sign of subclinical vitamin D deficiency". The Journal of Clinical Endocrinology & Metabolism. 93 (5): 1784–8. doi:10.1210/jc.2007-2254. PMID 18270256.
- Bender, David A. Nutritional Biochemistry of the Vitamins. Cambridge University Press. p. 99.
- Cherniack EP, Levis S, Troen BR (April 2008). "Hypovitaminosis D: a widespread epidemic". Geriatrics. 63 (4): 24–30. PMID 18376898.
- Winzenberg T, Jones G (February 2013). "Vitamin D and bone health in childhood and adolescence". Calcified Tissue International (Review). 92 (2): 140–50. doi:10.1007/s00223-012-9615-4. PMID 22710658. S2CID 17181183.
- Holick MF (October 2008). "Vitamin D: a D-Lightful health perspective". Nutrition Reviews. 66 (10 Suppl 2): S182–94. doi:10.1111/j.1753-4887.2008.00104.x. PMID 18844847.
- Polly, Patsie; Tan, Timothy C. (2014). "The role of vitamin D in skeletal and cardiac muscle function". Frontiers in Physiology. 5: 145. doi:10.3389/fphys.2014.00145. ISSN 1664-042X. PMC 3995052. PMID 24782788.
- Wang CJ, McCauley LK (December 2016). "Osteoporosis and Periodontitis". Current Osteoporosis Reports. 14 (6): 284–291. doi:10.1007/s11914-016-0330-3. PMC 5654540. PMID 27696284.
- Bakacak M, Serin S, Ercan O, Köstü B, Avci F, Kılınç M, Kıran H, Kiran G (15 September 2015). "Comparison of Vitamin D levels in cases with preeclampsia, eclampsia and healthy pregnant women". International Journal of Clinical and Experimental Medicine. 8 (9): 16280–6. PMC 4659033. PMID 26629145.
- Paterson CR, Ayoub D (October 2015). "Congenital rickets due to vitamin D deficiency in the mothers". Clinical Nutrition (Review). 34 (5): 793–8. doi:10.1016/j.clnu.2014.12.006. PMID 25552383.
- Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, et al. (February 2017). "Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data". BMJ. 356: i6583. doi:10.1136/bmj.i6583. PMC 5310969. PMID 28202713.
- Jolliffe DA, Greenberg L, Hooper RL, Mathyssen C, Rafiq R, de Jongh RT, Camargo CA, Griffiths CJ, Janssens W, Martineau AR (April 2019). "Vitamin D to prevent exacerbations of COPD: systematic review and meta-analysis of individual participant data from randomised controlled trials". Thorax. 74 (4): 337–345. doi:10.1136/thoraxjnl-2018-212092. PMID 30630893. S2CID 58548871.
- "Vitamin D deficiency increased risk of COVID in healthcare workers, new UK study shows". University of Birmingham. 7 October 2020. Retrieved 20 October 2020.
- Busby, Mattha (2021-01-10). "Does vitamin D combat Covid?". The Guardian. Retrieved 2021-01-10.
- Damascena, Alialdo Dantas; Azevedo, Laylla Mirella Galvão; Oliveira, Tarcio de Almeida; Santana, Jerusa da Mota; Pereira, Marcos (2021-08-12). "Addendum to vitamin D deficiency aggravates COVID-19: systematic review and meta-analysis". Critical Reviews in Food Science and Nutrition. 63 (4): 557–562. doi:10.1080/10408398.2021.1951652. ISSN 1549-7852. PMID 34384300. S2CID 236997712.
- Berridge MJ (1 February 2018). "Vitamin D deficiency: infertility and neurodevelopmental diseases (attention deficit hyperactivity disorder, autism, and schizophrenia)". American Journal of Physiology. Cell Physiology. 314 (2): C135–C151. doi:10.1152/ajpcell.00188.2017. PMID 29070492.
- Cirulli F, Musillo C, Berry A (5 February 2020). "Maternal Obesity as a Risk Factor for Brain Development and Mental Health in the Offspring". Neuroscience. 447: 122–135. doi:10.1016/j.neuroscience.2020.01.023. hdl:11573/1387747. PMID 32032668. S2CID 211029692.
- Kennel, Kurt, MD et al., Vitamin D Deficiency in Adults: When to Test and How to Treat, Mayo Clinic Proceedings, August 2010, pp752–758
- Janssen HC, Samson MM, Verhaar HJ (April 2002). "Vitamin D deficiency, muscle function, and falls in elderly people". The American Journal of Clinical Nutrition. 75 (4): 611–5. doi:10.1093/ajcn/75.4.611. PMID 11916748.
- "15 Negative Effects of Having a Low Body-fat Percentage". 2017-12-30.
- Dunnigan MG, Henderson JB (November 1997). "An epidemiological model of privational rickets and osteomalacia". The Proceedings of the Nutrition Society. 56 (3): 939–56. doi:10.1079/PNS19970100. PMID 9483661.
- Robertson I, Ford JA, McIntosh WB, Dunnigan MG (January 1981). "The role of cereals in the aetiology of nutritional rickets: the lesson of the Irish National Nutrition Survey 1943-8". The British Journal of Nutrition. 45 (1): 17–22. doi:10.1079/BJN19810073. PMID 6970590.
- Clements MR (1989). "The problem of rickets in UK Asians". Journal of Human Nutrition and Dietetics. 2 (2): 105–116. doi:10.1111/j.1365-277X.1989.tb00015.x.
- Pettifor JM (December 2004). "Nutritional rickets: deficiency of vitamin D, calcium, or both?". The American Journal of Clinical Nutrition. 80 (6 Suppl): 1725S–9S. doi:10.1093/ajcn/80.6.1725S. PMID 15585795.
- Weick MT (November 1967). "A history of rickets in the United States". The American Journal of Clinical Nutrition. 20 (11): 1234–41. doi:10.1093/ajcn/20.11.1234. PMID 4862158.
- Holick MF (December 2004). "Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease". The American Journal of Clinical Nutrition. 80 (6 Suppl): 1678S–88S. doi:10.1093/ajcn/80.6.1678S. PMID 15585788.
- Garrison, R., Jr., Somer, E., The nutrition desk reference(1997)
- DuPuis EM (2002). Nature's Perfect Food: How Milk Became America's Drink. NYU Press. ISBN 978-0-8147-1938-1.
- Nabeta HW, Kasolo J, Kiggundu RK, Kiragga AN, Kiguli S (September 2015). "Serum vitamin D status in children with protein-energy malnutrition admitted to a national referral hospital in Uganda". BMC Research Notes. 8: 418. doi:10.1186/s13104-015-1395-2. PMC 4562347. PMID 26346815.
- Pereira-Santos M, Costa PR, Assis AM, Santos CA, Santos DB (April 2015). "Obesity and vitamin D deficiency: a systematic review and meta-analysis". Obesity Reviews. 16 (4): 341–9. doi:10.1111/obr.12239. PMID 25688659. S2CID 6729646.
- Alpert PT, Shaikh U (October 2007). "The effects of vitamin D deficiency and insufficiency on the endocrine and paracrine systems". Biological Research for Nursing. 9 (2): 117–29. doi:10.1177/1099800407308057. PMID 17909164. S2CID 42930710.
- Norval M, Wulf HC (October 2009). "Does chronic sunscreen use reduce vitamin D production to insufficient levels?". The British Journal of Dermatology. 161 (4): 732–6. doi:10.1111/j.1365-2133.2009.09332.x. PMID 19663879. S2CID 12276606.
- Nowson CA, Margerison C (August 2002). "Vitamin D intake and vitamin D status of Australians". The Medical Journal of Australia. 177 (3): 149–52. doi:10.5694/j.1326-5377.2002.tb04702.x. PMID 12149085. S2CID 20278782.
- Bandgar TR, Shah NS (September 2010). "Vitamin D and hip fractures: Indian scenario". The Journal of the Association of Physicians of India. 58 (September 2010): 535–7. PMID 21391371. Archived from the original on 2016-03-04. Retrieved 2010-09-15.
Social and religious customs that require people to wear concealing clothing, veiling and traditional attire, such as the burqa, salvar kameez, and sari significantly prevents sun exposure.
- Cashman KD (April 2007). "Vitamin D in childhood and adolescence". Postgraduate Medical Journal (Review). 83 (978): 230–5. doi:10.1136/pgmj.2006.052787. PMC 2600028. PMID 17403948.
- Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, El-Hajj Fuleihan G, Josse RG, Lips P, Morales-Torres J (November 2009). "Global vitamin D status and determinants of hypovitaminosis D" (PDF). Osteoporosis International. 20 (11): 1807–20. doi:10.1007/s00198-009-0954-6. hdl:1871/27487. PMID 19543765. S2CID 52858668.
- Nair, Rathish; Maseeh, Arun (2012). "Vitamin D: The "sunshine" vitamin". Journal of Pharmacology & Pharmacotherapeutics. 3 (2): 118–126. doi:10.4103/0976-500X.95506 (inactive 1 August 2023). ISSN 0976-500X. PMC 3356951. PMID 22629085.
{{cite journal}}: CS1 maint: DOI inactive as of August 2023 (link) - Caruso R, Pallone F, Stasi E, Romeo S, Monteleone G (December 2013). "Appropriate nutrient supplementation in celiac disease". Annals of Medicine (Review). 45 (8): 522–31. doi:10.3109/07853890.2013.849383. PMID 24195595. S2CID 11093737.
- Margulies SL, Kurian D, Elliott MS, Han Z (November 2015). "Vitamin D deficiency in patients with intestinal malabsorption syndromes--think in and outside the gut". Journal of Digestive Diseases (Review). 16 (11): 617–33. doi:10.1111/1751-2980.12283. PMID 26316334. S2CID 28570357.
- Chakhtoura MT, Nakhoul N, Akl EA, Mantzoros CS, El Hajj Fuleihan GA (April 2016). "Guidelines on vitamin D replacement in bariatric surgery: Identification and systematic appraisal". Metabolism. 65 (4): 586–97. doi:10.1016/j.metabol.2015.12.013. PMC 4792722. PMID 26833101.
- Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, Bjelakovic M, Gluud C (January 2014). "Vitamin D supplementation for prevention of mortality in adults". The Cochrane Database of Systematic Reviews. 1 (1): CD007470. doi:10.1002/14651858.CD007470.pub3. PMID 24414552.
- Putzu A, Belletti A, Cassina T, Clivio S, Monti G, Zangrillo A, Landoni G (April 2017). "Vitamin D and outcomes in adult critically ill patients. A systematic review and meta-analysis of randomized trials". Journal of Critical Care. 38: 109–114. doi:10.1016/j.jcrc.2016.10.029. PMID 27883968. S2CID 24923003.
- "Vitamin D: On the double". American Academy of Pediatrics. 19 September 2016. Retrieved 10 April 2022.
- Sunyecz JA (August 2008). "The use of calcium and vitamin D in the management of osteoporosis". Therapeutics and Clinical Risk Management. 4 (4): 827–36. doi:10.2147/tcrm.s3552. PMC 2621390. PMID 19209265.
- Kennel KA, Drake MT, Hurley DL (August 2010). "Vitamin D deficiency in adults: when to test and how to treat". Mayo Clinic Proceedings. 85 (8): 752–7, quiz 757–8. doi:10.4065/mcp.2010.0138. PMC 2912737. PMID 20675513.
- Heaney RP (September 2011). "Assessing vitamin D status". Current Opinion in Clinical Nutrition and Metabolic Care. 14 (5): 440–4. doi:10.1097/MCO.0b013e328348ed85. PMID 21832900. S2CID 43800943.
- Alshahrani F, Aljohani N (September 2013). "Vitamin D: deficiency, sufficiency and toxicity". Nutrients. 5 (9): 3605–16. doi:10.3390/nu5093605. PMC 3798924. PMID 24067388.
- "Final Recommendation Statement: Vitamin D Deficiency: Screening - US Preventive Services Task Force". www.uspreventiveservicestaskforce.org. Retrieved 2017-12-01.
- Holick MF (July 2007). "Vitamin D deficiency". The New England Journal of Medicine. 357 (3): 266–81. doi:10.1056/NEJMra070553. PMID 17634462. S2CID 18566028.
- Holick MF (February 2002). "Vitamin D: the underappreciated D-lightful hormone that is important for skeletal and cellular health". Current Opinion in Endocrinology, Diabetes and Obesity. 9 (1): 87–98. doi:10.1097/00060793-200202000-00011. S2CID 87725403.
- Lee E, Koo J, Berger T (2014-01-24). "UVB phototherapy and skin cancer risk: a review of the literature". Int. J. Dermatol. 44 (5): 355–60. doi:10.1111/j.1365-4632.2004.02186.x. PMID 15869531. S2CID 11332443.
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D Calcium; Ross, A. C.; Taylor, C. L.; Yaktine, A. L.; Del Valle, H. B. (2011). Dietary Reference Intakes for Calcium and Vitamin D : Health and Medicine Division. doi:10.17226/13050. ISBN 978-0-309-16394-1. PMID 21796828. S2CID 58721779. Retrieved 2017-12-14.
{{cite book}}:|website=ignored (help) - "Federal Register May 27, 2016 Food Labeling: Revision of the Nutrition and Supplement Facts Labels. FR page 33982" (PDF).
- "Vitamin D supplementation: Recommendations for Canadian mothers and infants". Canadian Paediatric Society. Archived from the original on 2017-12-15. Retrieved 2017-12-14.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM (July 2011). "Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline". The Journal of Clinical Endocrinology & Metabolism. 96 (7): 1911–30. doi:10.1210/jc.2011-0385. PMID 21646368.
- Lee JY, So TY, Thackray J (October 2013). "A review on vitamin d deficiency treatment in pediatric patients". The Journal of Pediatric Pharmacology and Therapeutics (Review). 18 (4): 277–91. doi:10.5863/1551-6776-18.4.277. PMC 3979050. PMID 24719588.
- Tripkovic L, Lambert H, Hart K, Smith CP, Bucca G, Penson S, Chope G, Hyppönen E, Berry J, Vieth R, Lanham-New S (June 2012). "Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis". The American Journal of Clinical Nutrition. 95 (6): 1357–64. doi:10.3945/ajcn.111.031070. PMC 3349454. PMID 22552031.
- Moyad MA (2009). "Vitamin D: a rapid review". Dermatology Nursing. 21 (1): 25–30, 55. PMID 19283958. Section Dosage of Vitamin D Needed To Achieve 35 to 40 ng/mL (90–100 nmol/L). Re-published from Moyad MA (October 2008). "Vitamin D: a rapid review". Urologic Nursing (Review). 28 (5): 343–9, 384, quiz 350. PMID 18980100.
- van Groningen L, Opdenoordt S, van Sorge A, Telting D, Giesen A, de Boer H (April 2010). "Cholecalciferol loading dose guideline for vitamin D-deficient adults". European Journal of Endocrinology. 162 (4): 805–11. doi:10.1530/EJE-09-0932. PMID 20139241.
- Souberbielle JC, Cormier C, Cavalier E, et al. (2019). "Vitamin D supplementation in France in patients with or at risk for osteoporosis: recent data and new practices" (PDF). Joint Bone Spine. 87 (1): 25–29. doi:10.1016/j.jbspin.2019.04.004. PMID 31051244. S2CID 145023744.
- Ish Shalom S, Segal E, Salganik T, Raz B, Bromberg IL, Vieth R (2008). "Comparison of daily, weekly, and monthly vitamin D3 in ethanol dosing protocols for two months in elderly hip fracture patients". The Journal of Clinical Endocrinology & Metabolism. 93 (9): 3430–3435. doi:10.1210/jc.2008-0241. PMID 18544622.
- Takacs I, Toth BE, Szekeres L, Szabo B, Bakos B, Lakatos P (2016). "Randomized clinical trial to comparing the efficacy of daily, weekly and monthly administration of vitamin D3". Endocrine. 55 (55): 60–65. doi:10.1007/s12020-016-1137-9. PMID 27718150. S2CID 207364220.
- Chel V, Wijnhoven HA, Smit JH, Ooms M, Lips P (2008). "Efficacy of different doses and time intervals of oral vitamin D supplementation with or without calcium in elderly nursing home residents". Osteoporos. Int. 19 (5): 663–671. doi:10.1007/s00198-007-0465-2. PMC 2277446. PMID 17874029.
- De Niet S, Coffiner M, Da Silva S, Jandrain B, Souberbielle JC, Cavalier E (2018). "A Randomized study to compare a monthly to a daily Administration of Vitamin D₃ Supplementation". Nutrients. 10 (6): 659. doi:10.3390/nu10060659. PMC 6024703. PMID 29882841.
- Raimundo FV, et al. (2011). "Effect of high- versus low-fat meal on serum 25-hydroxyvitamin D levels after a single dose of vitamin D: a single-blind, parallel, randomized trial". International Journal of Endocrinology. 2011: 809069. doi:10.1155/2011/809069. PMC 3235461. PMID 22190928.
- Raimundo FV, et al. (2015). "Effect of fat on serum 25-hydroxyvitamin D3 levels after a single oral dose of vitamin D in young healthy adults: a double-blind randomized placebo-controlled study". Eur J Nutr. 54 (3): 391–6. doi:10.1007/s00394-014-0718-8. PMID 24853643. S2CID 36041502.
- Mulligan GB, et al. (2010). "Taking Vitamin D With the Largest Meal Improves Absorption and Results in Higher Serum Levels of 25-Hydroxyvitamin D3". Journal of Bone and Mineral Research. 25 (4): 928–930. doi:10.1002/jbmr.67. PMID 20200983. S2CID 22635542.
- Niramitmahapanya S, et al. (2011). "Type of dietary fat is associated with the 25-hydroxyvitaminD increment in response to vitamin D supplementation". The Journal of Clinical Endocrinology & Metabolism. 10 (96): 3170–3174. doi:10.1210/jc.2011-1518. PMC 3200243. PMID 21816779.
- Cavalier E, Jandrain B, Coffiner M, Da Silva S, De Niet S, Vanderbist F, Souberbielle JC (2016). "A Randomised, Cross-Over Study to Estimate the Influence of Food on the 25-Hydroxyvitamin D3 Serum Level after Vitamin D3 Supplementation". Nutrients. 5 (8): 309. doi:10.3390/nu8050309. PMC 4882721. PMID 27213447.
- Holick MF (November 2005). "The vitamin D epidemic and its health consequences". The Journal of Nutrition. 135 (11): 2739S–48S. doi:10.1093/jn/135.11.2739S. PMID 16251641.
- Kuang X, Liu C, Guo X, Li K, Deng Q, Li D (April 2020). "The combination effect of vitamin K and vitamin D on human bone quality: a meta-analysis of randomized controlled trials". Food & Function. 11 (4): 3280–3297. doi:10.1039/c9fo03063h. PMID 32219282. S2CID 214680203.
- Capozzi A, Scambia G, Lello S (October 2020). "Calcium, vitamin D, vitamin K2, and magnesium supplementation and skeletal health". Maturitas. 140: 55–63. doi:10.1016/j.maturitas.2020.05.020. PMID 32972636. S2CID 219785752.
- Levy DS, Grewal R, Le TH (October 2020). "Vitamin K deficiency: an emerging player in the pathogenesis of vascular calcification and an iatrogenic consequence of therapies in advanced renal disease". American Journal of Physiology. Renal Physiology. 319 (4): F618–F623. doi:10.1152/ajprenal.00278.2020. PMID 32830534. S2CID 221280657.
- Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF (September 2000). "Decreased bioavailability of vitamin D in obesity". The American Journal of Clinical Nutrition. 72 (3): 690–3. doi:10.1093/ajcn/72.3.690. PMID 10966885.
- Ogrodnik M, Jurk D (September 2017). "Senescence explains age- and obesity-related liver steatosis". Cell Stress. 1 (1): 70–72. doi:10.15698/cst2017.10.108. PMC 6551654. PMID 31225436.
- Hudges, Bess Dawson; Harris, Susan; Lichtenstein, Alice; Dolnikowski, Gregory; Rasmussen, Helen; Palermo, Nancy (2015). "Dietary fat increases vitamin D-3 absorption". Journal of the Academy of Nutrition and Dietetics. 115 (2): 225–230. doi:10.1016/j.jand.2014.09.014. PMID 25441954. Retrieved 3 October 2022.
- Ogrodnik M, Miwa S, Tchkonia T, Tiniakos D, Wilson CL, Lahat A, et al. (June 2017). "Cellular senescence drives age-dependent hepatic steatosis". Nature Communications. 8 (1): 15691. Bibcode:2017NatCo...815691O. doi:10.1038/ncomms15691. PMC 5474745. PMID 28608850.
- Chowdhury R, Kunutsor S, Vitezova A, Oliver-Williams C, Chowdhury S, Kiefte-de-Jong JC, Khan H, Baena CP, Prabhakaran D, Hoshen MB, Feldman BS, Pan A, Johnson L, Crowe F, Hu FB, Franco OH (April 2014). "Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies". BMJ. 348: g1903. doi:10.1136/bmj.g1903. PMC 3972416. PMID 24690623.
- Ginde AA, Liu MC, Camargo CA (March 2009). "Demographic differences and trends of vitamin D insufficiency in the US population, 1988-2004". Archives of Internal Medicine. 169 (6): 626–32. doi:10.1001/archinternmed.2008.604. PMC 3447083. PMID 19307527.
- "Vitamin D". NIH Office of Dietary Supplements. 11 February 2016. Retrieved 6 December 2016.
- Hoel DG, Berwick M, de Gruijl FR, Holick MF (19 October 2016). "The risks and benefits of sun exposure 2016". Dermato-Endocrinology. 8 (1): e1248325. doi:10.1080/19381980.2016.1248325. PMC 5129901. PMID 27942349.
- Ross AC, Taylor CL, Yaktine AL, Del Valle HB (2011). Dietary Reference Intakes for Calcium and Vitamin D. Washington, D.C: National Academies Press. doi:10.17226/13050. ISBN 978-0-309-16394-1. PMID 21796828. S2CID 58721779.
- "Vitamin D Status: United States, 2001–2006" (PDF). CDC NCHS Data Brief (59). March 2011.
- Rajakumar K (August 2003). "Vitamin D, cod-liver oil, sunlight, and rickets: a historical perspective". Pediatrics. 112 (2): e132–5. CiteSeerX 10.1.1.542.1645. doi:10.1542/peds.112.2.e132. PMID 12897318.
- Buttigliero C, Monagheddu C, Petroni P, Saini A, Dogliotti L, Ciccone G, Berruti A (2011). "Prognostic role of vitamin d status and efficacy of vitamin D supplementation in cancer patients: a systematic review". The Oncologist. 16 (9): 1215–27. doi:10.1634/theoncologist.2011-0098. PMC 3228169. PMID 21835895.
- Bolland MJ, Grey A, Gamble GD, Reid IR (April 2014). "The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis". The Lancet. Diabetes & Endocrinology. 2 (4): 307–20. doi:10.1016/S2213-8587(13)70212-2. PMID 24703049.
- Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, Bjelakovic M, Gluud C (January 2014). "Vitamin D supplementation for prevention of mortality in adults". The Cochrane Database of Systematic Reviews. 1 (1): CD007470. doi:10.1002/14651858.cd007470.pub3. PMID 24414552.
- Eliades M, Spyrou E, Agrawal N, Lazo M, Brancati FL, Potter JJ, Koteish AA, Clark JM, Guallar E, Hernaez R (August 2013). "Meta-analysis: vitamin D and non-alcoholic fatty liver disease". Alimentary Pharmacology & Therapeutics. 38 (3): 246–54. doi:10.1111/apt.12377. PMID 23786213. S2CID 7719230.
- Wang X, Li W, Zhang Y, Yang Y, Qin G (2015). "Association between vitamin D and non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: results from a meta-analysis". International Journal of Clinical and Experimental Medicine. 8 (10): 17221–34. PMC 4694215. PMID 26770315.
- Prietl, Barbara; Treiber, Gerlies; Pieber, Thomas; Amrein, Karin (2013). "Vitamin D and immune function". Nutrients. 5 (7): 2502–2521. doi:10.3390/nu5072502. PMC 3738984. PMID 23857223.
- "Does Vitamin D Prevent Autoimmune Disease? | Science-Based Medicine". sciencebasedmedicine.org. 2022-09-13. Retrieved 2022-11-04.
- Akbari S, Khodadadi B, Ahmadi SA, Abbaszadeh S, Shahsavar F (April 2018). "Association of vitamin D level and vitamin D deficiency with risk of preeclampsia: A systematic review and updated meta-analysis". Taiwanese Journal of Obstetrics & Gynecology. 57 (2): 241–247. doi:10.1016/j.tjog.2018.02.013. PMID 29673668.