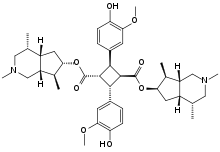
Incarvillateine
Incarvillateine is a complex monoterpene alkaloid that is a derivative of α-truxillic acid. It can be isolated from the plant genus Incarvillea.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Bis[(4R,4aS,6R,7S,7aR)-2,4,7-trimethyloctahydro-1H-cyclopenta[c]pyridin-6-yl] (1R,2R,3S,4S)-2,4-bis(4-hydroxy-3-methoxyphenyl)cyclobutane-1,3-dicarboxylate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C42H58N2O8 | |
| Molar mass | 718.932 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Biological activity
Opioidergic
Incarvillateine isolated from Incarvillea sinensis has demonstrated significant analgesic activity when compared to the opiate alkaloid morphine.[1]
Incarvillateine's pain-killing effect was partially blocked by administration of naloxone,[2] norbinaltorphimine and beta-funaltrexamine,[3] which are receptor antagonists with varying selectivity for mu and kappa opioid receptors. Naltrindole, a delta opioid receptor antagonist, did not counteract the analgesic activity of incarvillateine.[3]
These findings indicate that incarvillateine may possess opioidergic receptor activity, but it is worthy to note that some studies indicate that naloxone was ineffective at countering incarvillateine's analgesic activity.[4]
Adenosinergic
Incarvillateine's antinociceptive effect was blocked by the administration of adenosine receptor antagonists such as theophylline. This suggests that incarvillateine's main mechanism of action is mediated through the adenosine receptor.[4]
References
- Nakamura, M.; Chi, Y. M.; Yan, W. M.; Nakasugi, Y.; Yoshizawa, T.; Irino, N.; Hashimoto, F.; Kinjo, J.; Nohara, T. (1999-09-01). "Strong antinociceptive effect of incarvillateine, a novel monoterpene alkaloid from Incarvillea sinensis". Journal of Natural Products. 62 (9): 1293–1294. doi:10.1021/np990041c. ISSN 0163-3864. PMID 10514316.
- Ichikawa, Masaya; Takahashi, Masaki; Aoyagi, Sakae; Kibayashi, Chihiro (2004). "Total Synthesis of (−)-Incarvilline, (+)-Incarvine C, and (−)-Incarvillateine". Journal of the American Chemical Society. 126 (50): 16553–16558. doi:10.1021/ja0401702. PMID 15600360.
- Chi, Yu-Ming; Nakamura, Motoyuki; Yoshizawa, Toyokichi; Zhao, Xi-Ying; Yan, Wen-Mei; Hashimoto, Fumio; Kinjo, Junei; Nohara, Toshihiro; Sakurada, Shinobu (2005-10-01). "Pharmacological study on the novel antinociceptive agent, a novel monoterpene alkaloid from Incarvillea sinensis". Biological & Pharmaceutical Bulletin. 28 (10): 1989–1991. doi:10.1248/bpb.28.1989. ISSN 0918-6158. PMID 16204962.
- Wang, Mei-Liang; Yu, Gang; Yi, Shou-Pu; Zhang, Feng-Ying; Wang, Zhi-Tong; Huang, Bin; Su, Rui-Bin; Jia, Yan-Xing; Gong, Ze-Hui (2015-11-03). "Antinociceptive effects of incarvillateine, a monoterpene alkaloid from Incarvillea sinensis, and possible involvement of the adenosine system". Scientific Reports. 5: 16107. Bibcode:2015NatSR...516107W. doi:10.1038/srep16107. ISSN 2045-2322. PMC 4630779. PMID 26527075.