Indane-1,2,3-trione
Indane-1,2,3-trione is the organic compound with the formula C6H4(CO)3. The compound is the dehydrated derivative of C6H4(CO)2C(OH)2, known as ninhydrin, which is used to reveal fingerprints.
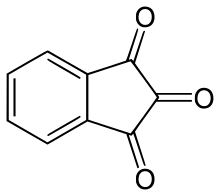 | |
| Names | |
|---|---|
| IUPAC name
Indane-1,2,3-trione | |
| Other names
Indanetrione | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H4O3 | |
| Molar mass | 160.128 g·mol−1 |
| Appearance | white powder |
| Density | 1.482 g/cm3 |
| Boiling point | 338.4 °C (641.1 °F; 611.5 K) |
| Hazards | |
| GHS labelling:[1] | |
 | |
| Warning | |
| H302, H315, H319, H335 | |
| P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Indane-1,2,3-trione, which reacts readily with nucleophiles (including water). Whereas for most carbonyl compounds, a carbonyl form is more stable than a product of water addition (hydrate), ninhydrin forms a stable hydrate of the central carbon because of the destabilizing effect of the adjacent carbonyl groups.
To generate the ninhydrin chromophore (2-(1,3-dioxoindan-2-yl)iminoindane-1,3-dione), the amine must condense to give a Schiff base. The reaction of ninhydrin with secondary amines gives an iminium salt, which is also coloured, generally being yellow–orange.
References
- "Indan-1,2,3-trione". pubchem.ncbi.nlm.nih.gov. Retrieved 4 February 2022.