Induced ovulation (animals)
Induced ovulation is when a female animal ovulates due to an externally-derived stimulus during, or just prior to, mating, rather than ovulating cyclically or spontaneously. Stimuli causing induced ovulation include the physical act of coitus or mechanical stimulation simulating this, sperm and pheromones.
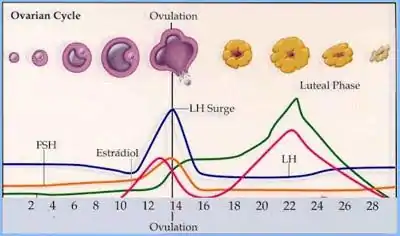
Ovulation occurs at the ovary surface and is described as the process in which an oocyte (female germ cell) is released from the follicle. Ovulation is a non-deleterious 'inflammatory response' which is initiated by a luteinizing hormone (LH) surge.[1] The mechanism of ovulation varies between species. In humans the ovulation process occurs around day 14 of the menstrual cycle, this can also be referred to as 'cyclical spontaneous ovulation'. However the monthly menstruation process is typically linked to humans and primates,[2] all other animal species ovulate by various other mechanisms.
Spontaneous ovulation is the ovulatory process in which the maturing ovarian follicles secrete ovarian steroids to generate pulsatile GnRH (the neuropeptide which controls all vertebrate reproductive function) release into the median eminence (the area which connects the hypothalamus to the anterior pituitary gland) to ultimately cause a pre-ovulatory LH surge. Spontaneously ovulating species go through menstrual cycles and are fertile at certain times based on what part of the cycle they are in. Species in which the females are spontaneous ovulators include rats, mice, guinea pigs, horse, pigs, sheep, monkeys, and humans.[3][4]
Induced ovulation is the process in which the pre-ovulatory LH surge and therefore ovulation is induced by some component of coitus e.g. receipt of genital stimulation. Usually, spontaneous steroid-induced LH surges are not observed in induced ovulator species throughout their reproductive cycles, which indicates that GnRH release is absent or reduced due to lack of positive feedback action from steroid hormones. However, by contradiction, some spontaneously ovulating species can occasionally undergo mating-induced preovulatory LH surges. Species in which the females are induced ovulators include cats, rabbits, ferrets, and camels.[3] In 1985, Chen et al., used Bactrian camels to investigate the factor(s) that induce ovulation during breeding season. They monitored the camel ovaries for ovulation by rectal palpation following insemination of semen samples. Chen et al., concluded that in this particular camel species ovulation was induced by the seminal plasma, and not by the spermatozoa.[5]
Evolution
Although the evolution of these two types of ovulation is not well understood, the type of ovulation that would be advantageous in some species can be understood by looking at social groups. Animals that have large, complex social groups benefit from spontaneous ovulation as only the best males get to breed with females. If there are few males suitable for breeding it makes sense to spread out the times at which females are fertile, therefore increasing the proportion in which conception occurs.[6] This does not explain the evolution of ovulation in all species however, for example some species appear to show estrus synchronisation.
Mechanism
In spontaneous ovulators, estrogen and progesterone secreted by the follicles as they grow and mature affects the release of GnRH, and therefore causes an LH surge. The LH surge then causes the release of the egg.
Ovulation is triggered in induced ovulators by an LH surge from the anterior pituitary that is induced during mating. Animals this has been recorded in include rabbits, voles, ferrets and camels.[3] In some species such as the ferret, the duration of intromission has no effect on the LH surge, whereas in other species such as the cat these are related and higher levels of LH were produced by mating multiple times. In many species, for a LH surge to occur, little intromission is required.
The pathways in which information reaches the brain and causes GnRH release are not understood well; however, midbrain and brainstem noradrenergic neurones appear to be activated in response to intromission during mating. These neurones then go on to stimulate the mediobasal hypothalamic to release GnRH from the median eminence.[3] Most experiments on GnRH and LH release have been focused on spontaneous ovulators, though there have been studies completed on some induced ovulators (e.g., rabbits, ferrets). From this, it appears that norepinephrine facilitates GnRH release in the rabbit and ferret and the locus coereuleus which is the part of the brain involved in conveying genital-somatosensory information to the GnRH neurones.[7] Other substances that have similar effects include neuropeptide Y.
Species
Many species have been found to be induced ovulators and the reasons for this are not always clear. However, one possible reason is that induced ovulation could provide a better reproductive potential for those species that typically have shorter life spans and less encounters resulting in lower mating opportunities throughout their lifetime.[8] Other species may be 'facultatively-induced ovulators' meaning that while they can spontaneously ovulate, the cycle may speed up or slow down depending on the presence of males, females or mating.[9]
Some rodents such as squirrels[9] and mole-rats are known induced ovulators. In rats the East African mole rat and the Cape-Dune, Natal, Highvield and blind mole rats are known induced ovulators. These species require mating to stimulate the vagina and cervix, resulting in ovulation in the females. The East African mole rat has been found to have small spines on its penis which are also thought to contribute to this stimulation of induced ovulation.[8]
The koala species are a lesser-known induced ovulator. The koalas require mating in which the presence of ejaculated semen is needed to stimulate the female to produce a LH surge (which would cause ovulation of a follicle). Unlike many other animals, simply being in the presence of a male koala is not enough to induce ovulation itself, nor is vaginal stimulation on its own sufficient to cause induced ovulation to occur.[10]
Cats are another widely-known induced ovulator. After mating, the LH levels in female cats surge, and the time to ovulation can be predicted to occur between 1–2 days later.[11]
Wolverines are other known induced ovulators which require physical mating to cause ovulation.[12]
Induced ovulation occurs in various carnivoran species,[13] including most felids[14] and several species of mustelids.[15] Many bear species are able to have induced ovulation including the grizzly bear, black bear and polar bear where both the presence of a male and mating itself are requirements for induced ovulation. However, there are some suggestions that mating is not as strict a requirement for ovulation in bears.[16]
Japanese black bears are induced ovulators. It was observed that most females kept separate from males did not ovulate, whereas females kept in areas with male bears did. Mating between the bears caused elevated progesterone levels, and this was seen by increased progesterone levels measured in the bears in the months that followed the mating seasons.[17] In Japanese black bears, the presence of a male was enough to cause a notable rise in progesterone levels even without mating. This could suggest that pheromonal/chemosensory factors could also contribute to induced ovulation in some species.[17]
Induced ovulation is able to occur in some fish species. In China freshwater fish including a variety of carp types, bream and loach are able to be induced to ovulate by using agonists of dopamine. This induction of ovulation from drugs is able to cause a predictable ovulation period and is very beneficial to farming of these species.[18]
In cattle
The natural cycle of spontaneous ovulation occurs in species such as cows.[19] There is a great demand for ovulation to be induced in cattle, as it allows farmers to synchronize their cattle to ovulate at the same time, helping improve the efficiency of dairy farming.[20] Induced ovulation can be utilized during the warmer seasons to increase plasma progesterone and improve the fertility of the cattle.[21] However, ovulation can only be induced in cows with mature follicles and merely initiates lutenization, it does not reduce the time for ovulation.
There are a number of methods that are used to induce ovulation in cattle such as: artificial insemination, introducing a number of hormones such as chorionic gonadotrophin, hCG and LH. As well as injecting progesterone by intravaginal devices called PRIDs (progesterone-releasing intravaginal devices)[22]
In cats
Domestic cats are often described as induced ovulators. During intromission, the penis probably causes distension of the posterior vagina and induces release of gonadotropin releasing hormone (GnRH) from the hypothalamus via neuroendocrine reflexes. A surge of luteinising hormone (LH) occurs within minutes of mating. With multiple matings, the LH surge is greater and lasts longer than when only one mating occurs. There are reports of ovulation without mating in cats. Spontaneous ovulation not only occurs in cats, but occurs with some frequency. It appears that non-copulatory ovulation may be possible in response to a variety of visual, auditory or olfactory cues. It is more appropriate to consider domestic cats to be both an induced and spontaneous ovulator.[23]
In rabbits
It has been known since 1905[24] that domestic rabbits are physically induced ovulators, although they may also ovulate spontaneously. Early reports stated that simply having an oestrous doe in close proximity to a buck can induce ovulation, although there were no data presented in these early reports.[25]
In camelids
Dromedary camels (Camelus dromedarius), bactrian camels (Camelus bactrianus), llamas (Lama glama) and alpacas (Lama pacos) are all induced ovulators.[26][27]
Bactrian camel
Bactrian camels ovulate after insemination into the vagina; it is the seminal plasma, but not the spermatozoa, which induces ovulation. Ovulation occurs in 87% of females after insemination: 66% ovulate within 36 hours and the rest by 48 hours (the same as natural mating). The least amount of semen required to elicit ovulation is about 1.0 ml.[26]
Alpaca
In alpaca, follicles ovulate approximately 26 hours after coital stimulation. Mounting accompanied by intromission is necessary to provide adequate stimulation for LH release and subsequent ovulation.[28] Deposition of semen, which contains ovulation-inducing factor (OIF),[4] has been shown to increase the chance of pregnancy. Prolonged copulation, causing abrasion and inflammation of the uterus, may enhance absorption of OIF.
References
- Richards, J. S.; Hedin, L. (1988). "Molecular aspects of hormone action in ovarian follicular development, ovulation, and luteinization". Annual Review of Physiology. 50: 441–463. doi:10.1146/annurev.ph.50.030188.002301. ISSN 0066-4278. PMID 3288100.
- Strassmann, B. I. (June 1996). "The evolution of endometrial cycles and menstruation". The Quarterly Review of Biology. 71 (2): 181–220. doi:10.1086/419369. ISSN 0033-5770. PMID 8693059. S2CID 6207295.
- Bakker, J.; Baum, M. J. (July 2000). "Neuroendocrine regulation of GnRH release in induced ovulators". Frontiers in Neuroendocrinology. 21 (3): 220–262. doi:10.1006/frne.2000.0198. ISSN 0091-3022. PMID 10882541. S2CID 873489.
- Adams, G.P.; Ratto, M.H. (2012). "Ovulation-inducing factor in seminal plasma: A review". Animal Reproduction Science. 136 (3): 148–156. doi:10.1016/j.anireprosci.2012.10.004. PMID 23141951.
- Chen, B. X.; Yuen, Z. X.; Pan, G. W. (1985-07-01). "Semen-induced ovulation in the bactrian camel (Camelus bactrianus)". Journal of Reproduction and Fertility. 74 (2): 335–339. doi:10.1530/jrf.0.0740335. ISSN 1470-1626. PMID 3900379.
- Conaway, C. H. (June 1971). "Ecological adaptation and mammalian reproduction". Biology of Reproduction. 4 (3): 239–247. CiteSeerX 10.1.1.1014.8958. doi:10.1093/biolreprod/4.3.239. ISSN 0006-3363. PMID 5000279.
- Spies, H. G.; Pau, K. Y.; Yang, S. P. (February 1997). "Coital and estrogen signals: a contrast in the preovulatory neuroendocrine networks of rabbits and rhesus monkeys". Biology of Reproduction. 56 (2): 310–319. doi:10.1095/biolreprod56.2.310. ISSN 0006-3363. PMID 9116126.
- Katandukila, Jestina V.; Bennett, Nigel C. (May 2016). "Pattern of ovulation in the East African root rat (Tachyoryctes splendens) from Tanzania: induced or spontaneous ovulator?". Canadian Journal of Zoology. 94 (5): 345–351. doi:10.1139/cjz-2015-0217. hdl:2263/59996.
- Jöchle, Wolfgang (1973). "Coitus-induced ovulation". Contraception. 7 (6): 523–564. doi:10.1016/0010-7824(73)90023-1. ISSN 0010-7824.
- Johnston, S. D.; O'Callaghan, P.; Nilsson, K.; Tzipori, G.; Curlewis, J. D. (2004-11-01). "Semen-induced luteal phase and identification of a LH surge in the koala (Phascolarctos cinereus)". Reproduction. 128 (5): 629–634. doi:10.1530/rep.1.00300. ISSN 1470-1626. PMID 15509709.
- Shille, V. M.; Munrot, Coralie; Farmer, Susan Walker; Papkoff, H.; Stabenfeld, G. H. (1983-09-01). "Ovarian and endocrine responses in the cat after coitus". Journal of Reproduction and Fertility. 69 (1): 29–39. doi:10.1530/jrf.0.0690029. ISSN 1470-1626. PMID 6684161.
- Mead, Rodney A.; Bowles, Mark; Starypan, Greg; Jones, Mike (1993-01-01). "Evidence for pseudopregnancy and induced ovulation in captive wolverines (Gulo gulo)". Zoo Biology. 12 (4): 353–358. doi:10.1002/zoo.1430120405. ISSN 1098-2361.
- Larivière, Serge; Ferguson, Steven H. (2003). "Evolution of induced ovulation in North American carnivores". Journal of Mammalogy. 84 (3): 937–947. doi:10.1644/bme-003.
- de Morais, Rosana Nogueira. "Reproduction in small felid males." Biology, Medicine, and Surgery of South American Wild Animals (2008): 312.
- Mead, Rodney A., et al. "Evidence for pseudopregnancy and induced ovulation in captive wolverines (Gulo gulo)." Zoo Biology 12.4 (1993): 353-358.
- Chang, Geng-Ruei; Yang, Chieh-Chung; Hsu, Son-Haur; Lin, Chang; Chiu, Chun-Lung; Chan, Fang-Tse; Mao, Frank Chiahung (2011). "Fecal Reproductive Steroid Profiles for Monitoring Reproductive Patterns in Female Formosan Black Bears (Ursus thibetanus formosanus)". Annales Zoologici Fennici. 48 (5): 275–286. doi:10.5735/086.048.0502. S2CID 84068397.
- Okano, Tsukasa; Nakamura, Sachiko; Nakashita, Rumiko; Komatsu, Takeshi; Murase, Tetsuma; Asano, Makoto; Tsubota, Toshio (2006). "Incidence of Ovulation without Coital Stimuli in Captive Japanese Black Bears (Ursus thibetanus japonicus) based on Serum Progesterone Profiles". Journal of Veterinary Medical Science. 68 (10): 1133–1137. doi:10.1292/jvms.68.1133. PMID 17085899.
- Peter, Richard E.; Lin, Hao-Ren; Van Der Kraak, Glen (1988-11-01). "Induced ovulation and spawning of cultured freshwater fish in China: Advances in application of GnRH analogues and dopamine antagonists". Aquaculture. Induced Spawning of Asian Fishes. 74 (1): 1–10. doi:10.1016/0044-8486(88)90080-4.
- Bogle, O. A.; Ratto, M. H.; Adams, G. P. (2011-08-01). "Evidence for the conservation of biological activity of ovulation-inducing factor in seminal plasma". Reproduction. 142 (2): 277–283. doi:10.1530/REP-11-0042. ISSN 1470-1626. PMID 21652637.
- Wiltbank, Milo C.; Pursley, J. Richard (2014-01-01). "The cow as an induced ovulator: Timed AI after synchronization of ovulation". Theriogenology. 81 (1): 170–185. doi:10.1016/j.theriogenology.2013.09.017. PMID 24274420.
- De Rensis, F.; Valentini, R.; Gorrieri, F.; Bottarelli, E.; Lopez-Gatius, F. (2008-06-01). "Inducing ovulation with hCG improves the fertility of dairy cows during the warm season". Theriogenology. 69 (9): 1077–1082. doi:10.1016/j.theriogenology.2008.01.020. PMID 18374407.
- Yavas, Y.; Wallon, J.S. (2000-07-01). "Induction of ovulation in postpartum suckled beef cows: A review". Theriogenology. 54 (1): 1–23. doi:10.1016/S0093-691X(00)00322-8. ISSN 0093-691X. PMID 10990345.
- "Estrus". The University of Sydney. 2012. Retrieved September 12, 2014.
- Heape, W. (1905). "Ovulation and degeneration of ova in the rabbit". Proc. R. Soc. B. 76 (509): 260–268. doi:10.1098/rspb.1905.0019.
- Staples, R.E. (1967). "Behavioural induction of ovulation in the oestrous rabbit". J. Reprod. Fertil. 13 (3): 429–435. doi:10.1530/jrf.0.0130429. PMID 6067508.
- Chen, B.X.; Yuen, Z.X.; Pan, G.W. (1985). "Semen-induced ovulation in the bactrian camel (Camelus bactrianus)". J. Reprod. Fertil. 74 (2): 335–339. doi:10.1530/jrf.0.0740335. PMID 3900379.
- Bravo, P. W.; Skidmore, J. A.; Zhao, X. X. (2000). "Reproductive aspects and storage of semen in Camelidae" (PDF). Animal Reproduction Science. 62 (1): 173–193. doi:10.1016/s0378-4320(00)00158-5. PMID 10924824.
- Fernandez-Baca, S.; Madden, D.H.L.; Novoa, C. (1970). "Effect of different mating stimuli on induction of ovulation in the alpaca". J. Reprod. Fertil. 22 (2): 261–267. doi:10.1530/jrf.0.0220261. PMID 5464117.