Isomigrastatin
Isomigrastatin is an analogue of migrastatin, an organic compound that naturally occurs in the Streptomyces platensis bacteria. Isomigrastatin has shown promise as a drug in the treatment of cancer. A laboratory synthesis was reported in 2007.[1]
 | |
| Names | |
|---|---|
| IUPAC name
4-[7-(4-Hydroxy-5-methoxy-3-methyl-12-oxo-oxacyclododeca-6,10-dien-2-yl)-5-methyl-4-oxo-oct-6-enyl]-piperidine-2,6-dione | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C27H39NO7 | |
| Molar mass | 489.60 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Synthesis
Total synthesis
Migrastatin synthesis is a precursor of isomigrastatin. In order to synthesize isomigrastatin, reagent 11 and 15 need to be prepared.[2] Through LACDAC reaction, Luche reduction, aqueous Ferrier rearrangement and Epoxidation, reagent 6, 7, 8, 9, 10 are synthesized to 11. Aldehyde 12 reacts is alkyldenated by Witting reagent 13 to make 14, and 14 is hydrogenated to afford 15.
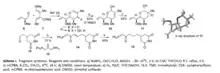
Fragment coupling of intermediate 11 and 15 would be the next step. Using lithium borohydride, lactol arrangement of reagent 11 is reduced to create alcohol 16 which could be converted to 17 as well. Coupled with phosphorane 15, reagent 16 and 17 are oxidized to synthesize aldehyde 18.
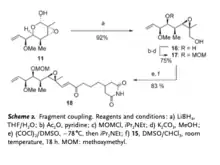
Enone 18 is then reduced by (S)-Me-CBS Corey catalyst to make intermediate 19, and lithium cyanomethylcuprate is added to make intermediate 21.
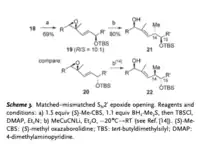
Acylation of alcohol 21 with racemic selenoacid 23 then leads to be intermediate 24, and ring-closing metathesis of 24 causes intermediate 26. Then it finally affords isomigrastatin by oxidative deselenation.
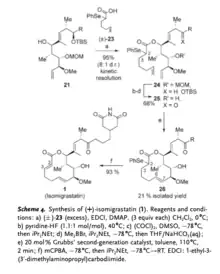
Biosynthesis
In terms of natural product, isomigrastatin is the polyketide that contains the glutarimide.[3] Biosynthesis of isomigrastatin starts with the PKS product 10 which is derived from S.platensis. PKS product that is lack of methyltransferase domain in module-5, a ketoreductase domain in module-8, and a KR and an enoylreductase domain in module-10 is needed to synthesize intermediate for isomigrastatin. On top of that, four tailoring steps are followed through intermediates. First, hydroxylation at C-8. Second, O-methylation at OHC-8. Third, dehydration at C-17 OH. Last, C-16 and C-17 olefin is reduced. PKS product 10 is then isolated to isomigrastatin.
References
- Krauss, IJ; et al. (2007). "Total Synthesis of (+)-Isomigrastatin". Angewandte Chemie. 46 (29): 5576–5579. doi:10.1002/anie.200701837. PMID 17583888.
- Krauss, Isaac J et al. “Total synthesis of (+)-isomigrastatin.” Angewandte Chemie (International ed. in English) vol. 46,29 (2007): 5576-9. doi:10.1002/anie.200701837
- Ma, M., Kwong, T., Lim, S. K., Ju, J., Lohman, J. R., & Shen, B. (2013). Post-polyketide synthase steps in iso-migrastatin biosynthesis, featuring tailoring enzymes with broad substrate specificity. Journal of the American Chemical Society, 135(7), 2489–2492. https://doi.org/10.1021/ja4002635