Isophthalonitrile
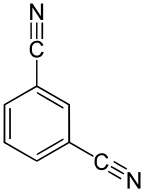
Isophthalonitrile is an organic compound with the formula C6H4(CN)2. Two other isomers exist, phthalonitrile and terephthalonitrile. All three isomers are produced commercially by ammoxidation of the corresponding xylene isomers. Isophthalonitrile is a colorless or white solid with low solubility in water.[1] Hydrogenation of isophthalonitrile affords m-xylylenediamine, a curing agent in epoxy resins and a component of some urethanes.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Benzene-1,3-dicarbonitrile | |
| Other names
1,3-Dicyanobenzene | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.009.940 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 2811 3276 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H4N2 | |
| Molar mass | 128.134 g·mol−1 |
| Melting point | 162–163 °C (324–325 °F; 435–436 K) |
| Boiling point | 288 °C (550 °F; 561 K) |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H302, H332 | |
| P261, P264, P270, P271, P301+P312, P304+P312, P304+P340, P312, P330, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Safety
LD50 (rat, oral) is 288 mg/kg.
References
- Pollak, Peter; Romeder, Gérard; Hagedorn, Ferdinand; Gelbke, Heinz-Peter (2000). "Nitriles". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_363.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.