Ixodes ricinus
Ixodes ricinus, the castor bean tick, is a chiefly European species of hard-bodied tick. It may reach a length of 11 mm (0.43 in) when engorged with a blood meal, and can transmit both bacterial and viral pathogens such as the causative agents of Lyme disease and tick-borne encephalitis.
| Ixodes ricinus | |
|---|---|
 | |
| Ixodes ricinus complete view (starved) | |
 | |
| Close-up view (engorged) | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Subphylum: | Chelicerata |
| Class: | Arachnida |
| Order: | Ixodida |
| Family: | Ixodidae |
| Genus: | Ixodes |
| Species: | I. ricinus |
| Binomial name | |
| Ixodes ricinus | |
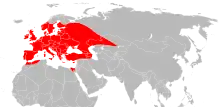 | |
| Range of I. ricinus (marked in red) in western Eurasia and North Africa | |
Description
In common with other species of Ixodes, I. ricinus has no eyes and is not ornate; it has no festoons (wrinkles along the posterior margin). The palpi are longer than they are wide, and an anal groove is above the anus. [1] It has a hard dorsal shield which covers the entire opisthosoma (abdomen), but only part of it in females and nymphs.[2] I. ricinus is the largest of the three common species of Ixodes in the British Isles (the other two being I. canisuga, the British dog tick, and I. trianguliceps, the vole tick). Adult males are 2.4–2.8 mm (0.09–0.11 in) long, and unfed nymphs are 1.3–1.5 mm (0.05–0.06 in) long; females are 3.0–3.6 mm (0.12–0.14 in) long before feeding and 11 mm (0.43 in) long when engorged.[3]
Distribution
Ixodes ricinus is found across Europe and into neighbouring parts of North Africa and the Middle East, extending as far north as Iceland and as far east as parts of Russia.[3] Its northern limit seems to be determined by environmental factors, including temperature, since a series of mild winters in Scandinavia coincided with an expansion northwards in the range of I. ricinus.[4]
I. ricinus is most frequent in habitats where its hosts are plentiful, including woodlands, heaths and forests.[3] It is most prevalent in relatively humid areas, and is absent from much of the Mediterranean Region where summers are dry.[5]
Role of climate change
As greenhouse gas emissions alter climatic conditions everywhere on Earth at a rapid rate, the range of conditions suitable for various species changes, and ixodes ricinus is no exception. Some research expects it to become 5–7% more prevalent on livestock farms in Great Britain, depending on the severity of climate change scenario followed.[6]
Lifecycle
Ixodes ricinus has a three-host lifecycle, which usually takes 2–3 years to complete, although it can take from 1 to 6 years in extreme cases.[3] Adults feed on large mammals such as sheep, cattle, dogs, deer, humans, and horses for 6–13 days, before dropping off. An engorged female lays several thousand eggs and subsequently dies.[3] The larvae that hatch do not actively seek a host, and usually feed on insectivores (order Eulipotyphla), although they may also find rodents, rabbits, birds, reptiles, or bats.[3][7] They feed for 3–5 days before dropping off and moulting. The resulting nymphs then ascend grasses or twigs to seek their next host, but must return to the moist microclimate at the soil surface if they become dehydrated.[8] The nymphs feed on small to medium-sized mammals.[5]
Disease transmission
A number of tick-borne diseases can be transmitted by I. ricinus to a variety of mammal hosts.[3] Dogs can be infected with Lyme disease (borreliosis), caused by the spirochaete bacteria Borrelia burgdorferi, B. afzelii, and B. garinii. Cattle can become infected with redwater fever (from the protozoans Babesia divergens, B. bovis, and B. ovis), Lyme disease (from B. burgdorferi), sheep tick pyemia (Staphylococcus aureus), cattle tick-borne fever (Anaplasma phagocytophila), Q fever (Coxiella burnetii), Boutonneuse fever (Rickettsia conorii), and the bacterium Anaplasma marginale. Horses may be infected with Lyme disease, Anaplasma phagocytophila, and the viral infection louping ill. Humans can become infected with Lyme disease, louping ill, Q fever, and tick-borne encephalitis,[3] and possibly sensitised to mammalian red meat, known as alpha-gal allergy.
Natural enemies
The parasitic wasp Ixodiphagus hookeri lays its eggs inside castor bean ticks, though the castor bean tick is not I. hookeri's sole host.
Taxonomic history
The scientific name of the castor bean tick dates back to the starting point of zoological nomenclature, the 1758 tenth edition of Carl Linnaeus' Systema Naturae, where it appeared as Acarus ricinus. Pierre André Latreille split the new genus Ixodes from Linnaeus' Acarus (which at that time contained all known ticks and mites), and I. ricinus was chosen as the type species.[9] It has subsequently been redescribed under a number of junior synonyms and subsequent combinations into different genera; these synonyms include Acarus ricinoides, Cynorhaestes reduvius, Cynorhaestes ricinus, Ixodes megathyreus, Ixodes bipunctatus, Cynorhaestes hermanni, Crotonus ricinus, Ixodes trabeatus, Ixodes plumbeus, Ixodes reduvius, Ixodes pustularum, Ixodes fodiens, Ixodes rufus, Ixodes sulcatus and Ixodes sciuri.[10]
See also
References
- Walker, M.D. (2018). "The Biology and Ecology of the Sheep Tick Ixodes ricinus" (PDF). Antenna: Royal Entomological Society. 42 (2): 61–65.
- Jaime Samour (2000). "Ticks". Avian medicine. Elsevier Health Sciences. pp. 223–224. ISBN 978-0-7234-2960-9.
- Frank L. Ruedisueli & Brigitte Manship. "Background information: Ixodes ricinus". University of Lincoln. Archived from the original on April 2, 2015. Retrieved July 22, 2010.
- Elisabet Lindgren, Lars Tälleklint & Thomas Polfeldt (2000). "Impact of climatic change on the northern latitude limit and population density of the disease-transmitting European tick Ixodes ricinus". Environmental Health Perspectives. 108 (2): 119–123. doi:10.2307/3454509. JSTOR 3454509. PMC 1637900. PMID 10656851.
- "Ixodes ricinus: European Castor Bean Tick, Castor Bean Tick, Sheep Tick" (PDF). Iowa State University. September 2009.
- Lihou, Katie; Wall, Richard (15 September 2022). "Predicting the current and future risk of ticks on livestock farms in Britain using random forest models". Veterinary Parasitology. 311: 109806. doi:10.1016/j.vetpar.2022.109806. PMID 36116333. S2CID 252247062.
- Mikula, P., Hromada, M., Koleničová, A., Pjenčák, P., Fulín, M., Olekšák, M., 2011. Prevalence of Ticks of birds in Slovak Karst. Folia oecologica presoviensis 5(4): 56-64.
- John L. Capinera (2008). "Ticks (Acari: Ixodida)". Encyclopedia of Entomology. Vol. 3 (2nd ed.). Springer. pp. 3733–3802. ISBN 978-1-4020-6242-1.
- Glen M. Kohls (1957). "Acarina: Ixodoidea" (PDF). Insects of Micronesia. 3 (3): 85–104.
- Edward Galton Wheler (1906). "British ticks". The Journal of Agricultural Science. 1 (4): 400–429. doi:10.1017/S0021859600000447.
External links
 Media related to Ixodes ricinus at Wikimedia Commons
Media related to Ixodes ricinus at Wikimedia Commons