Vomeronasal organ
The vomeronasal organ (VNO), or Jacobson's organ, is the paired auxiliary olfactory (smell) sense organ located in the soft tissue of the nasal septum, in the nasal cavity just above the roof of the mouth (the hard palate) in various tetrapods.[1] The name is derived from the fact that it lies adjacent to the unpaired vomer bone (from Latin vomer 'plowshare', for its shape) in the nasal septum. It is present and functional in all snakes and lizards, and in many mammals, including cats, dogs, cattle, pigs, and some primates. Some humans may have physical remnants of a VNO, but it is vestigial and non-functional.
| Vomeronasal organ | |
|---|---|
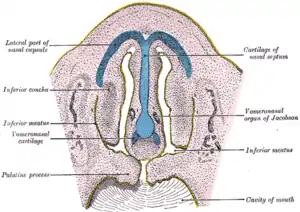 Frontal section of nasal cavities of a human embryo 28 mm long (Vomeronasal organ labeled at right) | |
| Details | |
| Precursor | Nasal placode |
| Lymph | Node |
| Identifiers | |
| Latin | organum vomeronasale |
| MeSH | D019147 |
| TA98 | A06.1.02.008 |
| TA2 | 3141 |
| FMA | 77280 |
| Anatomical terminology | |
The VNO contains the cell bodies of sensory neurons which have receptors that detect specific non-volatile (liquid) organic compounds which are conveyed to them from the environment. These compounds emanate from prey, predators, and the compounds called sex pheromones from potential mates. Activation of the VNO triggers an appropriate behavioral response to the presence of one of these three.
VNO neurons are activated by the binding of certain chemicals to their G protein-coupled receptors: they express receptors from three families, called V1R,[2][3][4] V2R, and FPR.[5][6] The axons from these neurons, called cranial nerve zero (CN 0), project to the accessory olfactory bulb, which targets the amygdala and bed nucleus of the stria terminalis, which in turn project to the anterior hypothalamus. These structures constitute the accessory olfactory system.
The VNO triggers the flehmen response in some mammals, which helps direct liquid organic chemicals to the organ. The VNO was discovered by Frederik Ruysch prior to 1732, and later by Ludwig Jacobson in 1813.[7]
Structure
The organ

The VNO is found at the base of the nasal cavity. It is split into two, being divided by the nasal septum, with both sides possessing an elongated C-shaped, or crescent, lumen. It is encompassed inside a bony or cartilaginous capsule which opens into the base of the nasal cavity.[8]
The system
The vomeronasal receptor neurons possess axons which travel from the VNO to the accessory olfactory bulb (AOB), which is also known as the vomeronasal bulb. These sensory receptors are located on the medial concave surface of the crescent lumen. The lateral, convex surface of the lumen is covered with non-sensory ciliated cells, where the basal cells are also found. At the dorsal and ventral aspect of the lumen are vomeronasal glands, which fill the vomeronasal lumen with fluid. Sitting next to the lumen are blood vessels that dilate or constrict, forming a vascular pump that deliver stimuli to the lumen. A thin duct, which opens onto the floor of the nasal cavity inside the nostril, is the only way of access for stimulus chemicals.
During embryological development, the vomeronasal sensory neurons form from the nasal (olfactory) placode, at the anterior edge of the neural plate (cranial nerve zero).
Sensory epithelium and receptors
The VNO is a tubular crescent shape and split into two pairs, separated by the nasal septum. The medial, concave area of the lumen is lined with a pseudo stratified epithelium that has three main cell types: receptor cells, supporting cells, and basal cells. The supporting cells are located superficially on the membrane while the basal cells are found on the basement membrane near the non-sensory epithelium. The receptor neurons possess apical microvilli, to which the sensory receptors are localized. These are G-protein-coupled receptors, which are often referred to as pheromone receptors since vomeronasal receptors have been tied to detecting pheromones.
Three G-protein-coupled receptors have been identified in the VNO, each found in distinct regions: the V1Rs, V2Rs, and FPRs. V1Rs, V2Rs and FPRs are seven transmembrane receptors which are not closely related to odorant receptors expressed in the main olfactory neuroepithelium.[9]
- V1 receptors, V1Rs, are linked to the G protein, Gαi2. The benefit of the GPCR is that they signal in more than one direction. V1Rs are located on the apical compartment of the VNO and a relatively short NH2 terminal and have a great sequence diversity in their transmembrane domains. V1R is specifically expressed in the rodent vomeronasal organ (VNO) and is thought to be responsible for pheromone reception, eliciting a signal transduction.[10]
- V2 receptors, V2Rs, are linked to the G-protein, Gαo. These have long extracellular NH2 terminals which are thought to be the binding domain for pheromonal molecules and are located on the basal compartment of the VNO. V2R genes can be grouped into four separate families, labelled A – D. Family C V2Rs are quite distinct from the other families, and they are expressed in most basal neurons of the VNO.
The vomeronasal organ's sensory neurons act on a different signaling pathway than that of the main olfactory system's sensory neurons. Activation of the receptors stimulates phospholipase C,[11] which in turn opens the ion channel TRPC2.[12][13] Upon stimulation activated by pheromones, IP3 production has been shown to increase in VNO membranes in many animals, while adenylyl cyclase and cyclic adenosine monophosphate (cAMP), the major signaling transduction molecules of the main olfactory system, remain unaltered. This trend has been shown in many animals, such as the hamster, the pig, the rat, and the garter snake upon introduction of vaginal or seminal secretions into the environment.
V1Rs and V2Rs are activated by distinct ligands or pheromones.
- Gi proteins are activated upon stimulation with lipophilic odorants.
- Go proteins are activated by nonvolatile proteins, such as the major urinary proteins in mice[14][15] and exocrine gland-secreting peptide 1 (ESP1).[16]
Many vomeronasal neurons are activated by chemicals in urine. Some of the active compounds are sulfated steroids.[17] Detecting the types and amounts of different sulfated steroids conveys information about the urine donor's physiological state, and may therefore serve as an honest signal.
Recent studies proved a new family of formyl peptide receptor like proteins in VNO membranes of mice, which points to a close phylogenetic relation of signaling mechanisms used in olfaction and chemosensors.[5]
Sensory neurons
Vomeronasal sensory neurons are extremely sensitive and fire action potentials at currents as low as 1 pA. Many patch-clamp recordings have confirmed the sensitivity of the vomeronasal neurons. This sensitivity is tied to the fact that the resting potential of the vomeronasal neurons is relatively close to that of the firing threshold of these neurons. Vomeronasal sensory neurons also show remarkably slow adaptation and the firing rate increases with increasing current up to 10 pA. The main olfactory sensory neurons fire single burst action potentials and show a much quicker adaptation rate. Activating neurons that have V1 receptors, V1Rs, cause field potentials that have weak, fluctuating responses that are seen the anterior of the accessory olfactory bulb, AOB. Activation of neurons that contain V2 receptors, V2Rs, however, promote distinct oscillations in the posterior of the AOB.[18]
Function
In mammals, the sensory neurons of the vomeronasal organ detect non-volatile chemical cues, which requires direct physical contact with the source of odor. Notably, some scents act as chemical-communication signals (pheromones) from other individuals of the same species. Unlike the main olfactory bulb that sends neuronal signals to the olfactory cortex, the VNO sends neuronal signals to the accessory olfactory bulb and then to the amygdala, BNST, and ultimately hypothalamus. Since the hypothalamus is a major neuroendocrine center (affecting aspects of reproductive physiology and behavior as well as other functions such as body temperature), this may explain how scents influence aggressive and mating behavior. For example, in many vertebrates, nerve signals from the brain pass sensory information to the hypothalamus about seasonal changes and the availability of a mate. In turn, the hypothalamus regulates the release of reproductive hormones required for breeding.[19] Some pheromones are detected by the main olfactory system.[20]
Animals which possess
The vomeronasal organ originated in tetrapods. The functional vomeronasal system is found in all snakes and lizards,[21] and many mammals.
- Salamanders perform a nose-tapping behavior to presumably activate their VNO.[22]
- Snakes use this organ to sense prey, sticking their tongue out to gather scents and touching it to the opening of the organ when the tongue is retracted.[23]
- The organ is well developed in strepsirrhine primates such as lemurs and lorises,[24] developed to varying degrees in New World monkeys, and underdeveloped in Old World monkeys and apes.[25]
- Elephants transfer chemosensory stimuli to the vomeronasal opening in the roof of their mouths using the prehensile structure, sometimes called a finger, at the tips of their trunks.[26]
- Painted turtles use this organ to use their sense of smell underwater.[26]
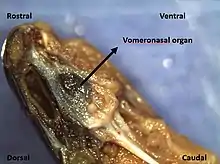
- Garter snakes – In addition to the main olfactory system, garter snakes also have the vomeronasal system which consists of the vomeronasal organ. The vomeronasal organ plays an important role in some vertebrates with its sensitivity toward chemicals that are related to mating or sensing prey. For example, the snakes use the organ to detect the presence of prey or predator by gathering the chemical cues in the environment through the flicking behavior of the forked tongue. Moreover, garter snakes also use the vomeronasal organ in their pheromone communication as well. Particularly, there should be a distinction made between the odors and vomodors. Odors are chemicals detected by the sensory cells in the nasal epithelium through the process of olfaction. Vomodors are chemicals detected by the sensory cells from the vomeronasal organ through the process of vomerolfaction.[27] Upon entering the lumen of the organ, the chemical molecules will come into contact with the sensory cells which are attached to the neurosensory epithelium of the vomeronasal organ. More importantly, a new research has demonstrated that the vomeronasal organ is necessary in order for garter snake to respond to airborne prey odors, but fail to respond to airborne non-prey odors.[28]
In some other mammals the entire organ contracts or pumps in order to draw in the scents.[29]

Flehmen response
Some mammals, particularly felids (cats) and ungulates (which includes horses, cattle, and pigs among other species), use a distinctive facial movement called the flehmen response to direct inhaled compounds to the VNO. The animal lifts its head after finding the odorant, wrinkles its nose while lifting its lips, and ceases to breathe momentarily.
Flehmen behavior is associated with "anatomical specialization", and animals that present flehmen behavior have incisive papilla and ducts, which connect the oral cavity to the VNO, that are found behind their teeth. However, horses are the exception: they exhibit flehmen response but do not have an incisive duct communication between the nasal and the oral cavity because they do not breathe through their mouths; instead, the VNOs connect to the nasal passages by the nasopalatine duct.[30]
Cats use their vomeronasal organ when scent rubbing; they are able to discriminate between similar smelling substances using this organ, and then perform the rubbing behaviour.[31]
Evidence for existence in humans
Many studies have tried to determine whether there is a VNO in adult human beings. Trotier et al.[32] estimated that around 92% of their subjects that had no septal surgery had at least one intact VNO. Kjaer and Fisher Hansen, on the other hand,[33] stated that the VNO structure disappears during fetal development as it does for some primates.[34] However, Smith and Bhatnagar (2000)[35] asserted that Kjaer and Fisher Hansen simply missed the structure in older fetuses. Won (2000) found evidence of a VNO in 13 of his 22 cadavers (59.1%) and 22 of his 78 living patients (28.2%).[36] In a study using retrospective analysis of nearly one thousand outpatient nasal endoscopies, Stoyanov et al. (2016) found the organ to be present in 26.83% of the Bulgarian population.[37]
Given these findings, some scientists have argued that there is a VNO in adult human beings.[38][39] However, most investigators have sought to identify the opening of the VNO in humans, rather than identify the tubular epithelial structure itself.[40] Thus it has been argued that such studies, employing macroscopic observational methods, have sometimes misidentified or even missed the vomeronasal organ.[40]
Among studies that use microanatomical methods, there is no reported evidence that human beings have active sensory neurons like those in working vomeronasal systems of other animals.[41] Furthermore, there is no evidence to date that suggests there are nerve and axon connections between any existing sensory receptor cells that may be in the adult human VNO and the brain.[42] Likewise, there is no evidence for any accessory olfactory bulb in adult human beings,[43] and the key genes involved in VNO function in other mammals have pseudogenized in human beings. Therefore, while many debate the structure's presence in adult human beings, a review of the scientific literature by Tristram Wyatt concluded that on current evidence, "most in the field... are skeptical about the likelihood of a functional VNO in adult human beings."[44]
History
The VNO was discovered by Frederik Ruysch prior to 1732, and later by Ludwig Jacobson in 1813.[7]
References
- Nakamuta S, Nakamuta N, Taniguchi K, Taniguchi K. Histological and ultrastructural characteristics of the primordial vomeronasal organ in lungfish. Anat Rec (Hoboken). 2012 Mar;295(3):481-91. doi: 10.1002/ar.22415. Epub 2012 Jan 23. PMID 22271496.
- Dulac C, Axel R (October 1995). "A novel family of genes encoding putative pheromone receptors in mammals". Cell. 83 (2): 195–206. doi:10.1016/0092-8674(95)90161-2. PMID 7585937. S2CID 18784638.
- Matsunami H, Buck LB (August 1997). "A multigene family encoding a diverse array of putative pheromone receptors in mammals". Cell. 90 (4): 775–784. doi:10.1016/s0092-8674(00)80537-1. PMID 9288756. S2CID 14898961.
- Ryba NJ, Tirindelli R (August 1997). "A new multigene family of putative pheromone receptors". Neuron. 19 (2): 371–379. doi:10.1016/S0896-6273(00)80946-0. hdl:11381/2435950. PMID 9292726. S2CID 18615918.
- Rivière S, Challet L, Fluegge D, Spehr M, Rodriguez I (May 2009). "Formyl peptide receptor-like proteins are a novel family of vomeronasal chemosensors". Nature. 459 (7246): 574–577. Bibcode:2009Natur.459..574R. doi:10.1038/nature08029. PMID 19387439. S2CID 4302009.
- Liberles SD, Horowitz LF, Kuang D, Contos JJ, Wilson KL, Siltberg-Liberles J, et al. (June 2009). "Formyl peptide receptors are candidate chemosensory receptors in the vomeronasal organ". Proceedings of the National Academy of Sciences of the United States of America. 106 (24): 9842–9847. Bibcode:2009PNAS..106.9842L. doi:10.1073/pnas.0904464106. PMC 2690606. PMID 19497865.
- Jacobson, L. (1813). Anatomisk Beskrivelse over et nyt Organ i Huusdyrenes Næse. Veterinær=Selskapets Skrifter [in Danish] 2,209–246.
- Meredith, Michael. "The Vomeronasal Organ". FSU Program in Neuroscience. Florida State University. Archived from the original on 2013-02-11. Retrieved 2013-05-27.
- Tirindelli R, Dibattista M, Pifferi S, Menini A (July 2009). "From pheromones to behavior". Physiological Reviews. 89 (3): 921–956. CiteSeerX 10.1.1.460.5566. doi:10.1152/physrev.00037.2008. PMID 19584317.
- Date-Ito A, Ohara H, Ichikawa M, Mori Y, Hagino-Yamagishi K (April 2008). "Xenopus V1R vomeronasal receptor family is expressed in the main olfactory system". Chemical Senses. 33 (4): 339–346. doi:10.1093/chemse/bjm090. PMID 18238827.
- Holy TE, Dulac C, Meister M (September 2000). "Responses of vomeronasal neurons to natural stimuli". Science. 289 (5484): 1569–1572. Bibcode:2000Sci...289.1569H. CiteSeerX 10.1.1.420.6387. doi:10.1126/science.289.5484.1569. PMID 10968796.
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G (February 2002). "Loss of sex discrimination and male-male aggression in mice deficient for TRP2". Science. 295 (5559): 1493–1500. Bibcode:2002Sci...295.1493S. doi:10.1126/science.1069259. PMID 11823606. S2CID 84419443.
- Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R (April 2002). "Altered sexual and social behaviors in trp2 mutant mice". Proceedings of the National Academy of Sciences of the United States of America. 99 (9): 6376–6381. Bibcode:2002PNAS...99.6376L. doi:10.1073/pnas.082127599. PMC 122956. PMID 11972034.
- "Aggression protein found in mice". BBC News. 5 December 2007. Retrieved 26 September 2009.
- Chamero P, Marton TF, Logan DW, Flanagan K, Cruz JR, Saghatelian A, et al. (December 2007). "Identification of protein pheromones that promote aggressive behaviour". Nature. 450 (7171): 899–902. Bibcode:2007Natur.450..899C. doi:10.1038/nature05997. PMID 18064011. S2CID 4398766.
- Kimoto H, Haga S, Sato K, Touhara K (October 2005). "Sex-specific peptides from exocrine glands stimulate mouse vomeronasal sensory neurons". Nature. 437 (7060): 898–901. Bibcode:2005Natur.437..898K. doi:10.1038/nature04033. PMID 16208374. S2CID 4388164.
- Nodari F, Hsu FF, Fu X, Holekamp TF, Kao LF, Turk J, Holy TE (June 2008). "Sulfated steroids as natural ligands of mouse pheromone-sensing neurons". The Journal of Neuroscience. 28 (25): 6407–6418. doi:10.1523/JNEUROSCI.1425-08.2008. PMC 2726112. PMID 18562612.
- Keverne EB (October 1999). "The vomeronasal organ". Science. 286 (5440): 716–720. doi:10.1126/science.286.5440.716. PMID 10531049.
- "Kimball, J.W. Pheromones. Kimball's Biology Pages. Sep 2008". Archived from the original on 2018-01-21. Retrieved 2008-11-01.
- Keller M, Baum MJ, Brock O, Brennan PA, Bakker J (June 2009). "The main and the accessory olfactory systems interact in the control of mate recognition and sexual behavior" (PDF). Behavioural Brain Research. 200 (2): 268–276. doi:10.1016/j.bbr.2009.01.020. hdl:2268/72698. PMID 19374011. S2CID 3997259.
- Baeckens S, Herrel A, Broeckhoven C, Vasilopoulou-Kampitsi M, Huyghe K, Goyens J, Van Damme R (September 2017). "Evolutionary morphology of the lizard chemosensory system". Scientific Reports. 7 (1): 10141. Bibcode:2017NatSR...710141B. doi:10.1038/s41598-017-09415-7. PMC 5583331. PMID 28871144.
- Dawley EM, Bass AH (May 1989). "Chemical access to the vomeronasal organs of a plethodontid salamander". Journal of Morphology. 200 (2): 163–174. doi:10.1002/jmor.1052000206. PMID 29865657. S2CID 46931736.
- Baeckens S, Van Damme R, Cooper WE (March 2017). "How phylogeny and foraging ecology drive the level of chemosensory exploration in lizards and snakes". Journal of Evolutionary Biology. 30 (3): 627–640. doi:10.1111/jeb.13032. hdl:10067/1396740151162165141. PMID 28009479. S2CID 32804222.
- Gould L, Sauther ML, Tattersall I, eds. (2006). "Chapter 1: Origin of the Malagasy Strepsirhine Primates". Lemurs: Ecology and Adaptation. Springer. pp. 3–18. ISBN 978-0-387-34585-7.
- Ankel-Simons F (2007). "Chapter 9: Sense Organs and Viscera". Primate Anatomy (3rd ed.). Academic Press. pp. 392–514. ISBN 978-0-12-372576-9.
- Simon VA (2010). Adaptations in the Animal Kingdom. Xlibris Corp. p. 31. ISBN 978-1450033640.
- Cooper WE, Burghardt GM (January 1990). "Vomerolfaction and vomodor". Journal of Chemical Ecology. 16 (1): 103–105. doi:10.1007/BF01021271. PMID 24264899. S2CID 26924795.
- Zuri I, Halpern M (February 2003). "Differential effects of lesions of the vomeronasal and olfactory nerves on garter snake (Thamnophis sirtalis) responses to airborne chemical stimuli". Behavioral Neuroscience. 117 (1): 169–183. doi:10.1037/0735-7044.117.1.169. PMID 12619919.
- Thewissen, J. G. M.; Nummela, Sirpa, eds. (2008). Sensory Evolution on the Threshold: Adaptations in Secondarily Aquatic Vertebrates. Berkeley: University of California Press. p. 45. ISBN 9780520252783.
- Briggs, Karen (2013-12-11). "Equine Sense of Smell". The Horse. Retrieved 2013-12-15.
- Griffith CA, Steigerwald ES, Buffington CA (October 2000). "Effects of a synthetic facial pheromone on behavior of cats". Journal of the American Veterinary Medical Association. 217 (8): 1154–1156. doi:10.2460/javma.2000.217.1154. PMID 11043684.
- Trotier D, Eloit C, Wassef M, Talmain G, Bensimon JL, Døving KB, Ferrand J (August 2000). "The vomeronasal cavity in adult humans". Chemical Senses. 25 (4): 369–380. doi:10.1093/chemse/25.4.369. PMID 10944499.
- Kjaer I, Fischer Hansen B (February 1996). "The human vomeronasal organ: prenatal developmental stages and distribution of luteinizing hormone-releasing hormone". European Journal of Oral Sciences. 104 (1): 34–40. doi:10.1111/j.1600-0722.1996.tb00043.x. PMID 8653495.
- Smith TD, Siegel MI, Bhatnagar KP (August 2001). "Reappraisal of the vomeronasal system of catarrhine primates: ontogeny, morphology, functionality, and persisting questions". The Anatomical Record. 265 (4): 176–192. doi:10.1002/ar.1152. PMID 11519019. S2CID 24546998.
- Smith TD, Bhatnagar KP (October 2000). "The human vomeronasal organ. Part II: prenatal development". Journal of Anatomy. 197 (3): 421–436. doi:10.1046/j.1469-7580.2000.19730421.x. PMC 1468143. PMID 11117628.
- Won J, Mair EA, Bolger WE, Conran RM (August 2000). "The vomeronasal organ: an objective anatomic analysis of its prevalence". Ear, Nose, & Throat Journal. 79 (8): 600–605. doi:10.1177/014556130007900814. PMID 10969469.
- Stoyanov G, Moneva K, Sapundzhiev N, Tonchev AB (April 2016). "The vomeronasal organ - incidence in a Bulgarian population". The Journal of Laryngology and Otology. 130 (4): 344–347. doi:10.1017/S0022215116000189. PMID 26831012. S2CID 1696242.
- Johnson A, Josephson R, Hawke M (April 1985). "Clinical and histological evidence for the presence of the vomeronasal (Jacobson's) organ in adult humans". The Journal of Otolaryngology. 14 (2): 71–79. PMID 4068105.
- Foltán R, Sedý J (January 2009). "Behavioral changes of patients after orthognathic surgery develop on the basis of the loss of vomeronasal organ: a hypothesis". Head & Face Medicine. 5: 5. doi:10.1186/1746-160X-5-5. PMC 2653472. PMID 19161592.
- Bhatnagar KP, Smith TD (September 2001). "The human vomeronasal organ. III. Postnatal development from infancy to the ninth decade". Journal of Anatomy. 199 (Pt 3): 289–302. doi:10.1046/j.1469-7580.2001.19930289.x. PMC 1468331. PMID 11554506.
- Witt M, Hummel T (2006). Vomeronasal versus olfactory epithelium: is there a cellular basis for human vomeronasal perception?. International Review of Cytology. Vol. 248. pp. 209–59. doi:10.1016/S0074-7696(06)48004-9. ISBN 9780123646521. PMID 16487792.
- Wysocki CJ, Preti G (November 2004). "Facts, fallacies, fears, and frustrations with human pheromones". The Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology. 281 (1): 1201–1211. doi:10.1002/ar.a.20125. PMID 15470677.
- Bhatnagar KP, Kennedy RC, Baron G, Greenberg RA (May 1987). "Number of mitral cells and the bulb volume in the aging human olfactory bulb: a quantitative morphological study". The Anatomical Record. 218 (1): 73–87. doi:10.1002/ar.1092180112. PMID 3605663. S2CID 25630359.
- Wyatt, Tristram D. (2003). Pheromones and Animal Behaviour: Communication by Smell and Taste. Cambridge: Cambridge University Press. ISBN 0-521-48526-6. p295
Further reading
- Døving KB, Trotier D (November 1998). "Structure and function of the vomeronasal organ". The Journal of Experimental Biology. 201 (Pt 21): 2913–2925. doi:10.1242/jeb.201.21.2913. PMID 9866877.
- Silvotti L, Moiani A, Gatti R, Tirindelli R (December 2007). "Combinatorial co-expression of pheromone receptors, V2Rs". Journal of Neurochemistry. 103 (5): 1753–1763. doi:10.1111/j.1471-4159.2007.04877.x. PMID 17854397. S2CID 11198963.
- Keverne EB (October 1999). "The vomeronasal organ". Science. 286 (5440): 716–720. doi:10.1126/science.286.5440.716. PMID 10531049.
- Meredith M (May 2001). "Human vomeronasal organ function: a critical review of best and worst cases". Chemical Senses. 26 (4): 433–445. doi:10.1093/chemse/26.4.433. PMID 11369678. S2CID 17248981.
- Evans CS (June 2006). "Accessory chemosignaling mechanisms in primates". American Journal of Primatology. 68 (6): 525–544. doi:10.1002/ajp.20250. PMID 16715503. S2CID 25874777.
- Wekesa KS, Anholt RR (August 1997). "Pheromone regulated production of inositol-(1, 4, 5)-trisphosphate in the mammalian vomeronasal organ". Endocrinology. 138 (8): 3497–3504. doi:10.1210/endo.138.8.5338. PMID 9231804.
- Monti-Bloch L, Jennings-White C, Berliner DL (November 1998). "The human vomeronasal system. A review". Annals of the New York Academy of Sciences. 855 (1): 373–389. Bibcode:1998NYASA.855..373M. doi:10.1111/j.1749-6632.1998.tb10595.x. PMID 9929629. S2CID 38973467.