Knorr quinoline synthesis
| Knorr quinoline synthesis | |
|---|---|
| Named after | Ludwig Knorr |
| Reaction type | Ring forming reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000394 |
The Knorr quinoline synthesis is an intramolecular organic reaction converting a β-ketoanilide to a 2-hydroxyquinoline using sulfuric acid. This reaction was first described by Ludwig Knorr (1859–1921) in 1886[1]
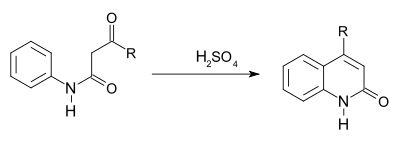
The reaction is a type of electrophilic aromatic substitution accompanied by elimination of water. A 1964 study found that with certain reaction conditions formation of a 4-hydroxyquinoline is a competing reaction.[2] For instance, the compound benzoylacetanilide (1) forms the 2-hydroxyquinoline (2) in a large excess of polyphosphoric acid (PPA) but 4-hydroxyquinoline 3 when the amount of PPA is small. A reaction mechanism identified a N,O-dicationic intermediate A with excess acid capable of ring-closing and a monocationic intermediate B which fragments to aniline and (ultimately to) acetophenone. Aniline reacts with another equivalent of benzoylacetanilide before forming the 4-hydroxyquinoline.

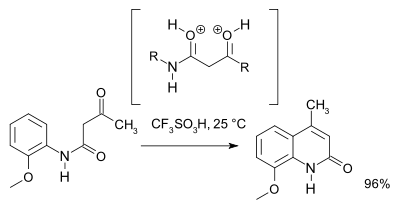
A 2007 study[3] revised the reaction mechanism and based on NMR spectroscopy and theoretical calculations favors an O,O-dicationic intermediate (a superelectrophile) over the N,O dicationic intermediate . For preparative purposes triflic acid is recommended:
References
- Synthetische Versuche mit dem Acetessigester Justus Liebig's Annalen der Chemie Volume 236, Issue 1–2, Date: 1886, Pages: 69–115 Ludwig Knorr doi:10.1002/jlac.18862360105
- The Conversion of Benzoylacetanilides into 2- and 4-Hydroxyquinolines B. Staskun J. Org. Chem. 1964; 29(5); 1153–1157. doi:10.1021/jo01028a038
- Knorr Cyclizations and Distonic Superelectrophiles Kiran Kumar Solingapuram Sai, Thomas M. Gilbert, and Douglas A. Klumpp J. Org. Chem. 2007, 72, 9761–9764 doi:10.1021/jo7013092