LRRIQ3
LRRIQ3 (Leucine-rich repeats and IQ motif containing 3), which is also known as LRRC44, is a protein that in humans is encoded by the LRRIQ3 gene.[5] It is predominantly expressed in the testes, and is linked to a number of diseases.[6]
| LRRIQ3 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | LRRIQ3, LRRC44, leucine-rich repeats and IQ motif containing 3, leucine rich repeats and IQ motif containing 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 617957 MGI: 1921685 HomoloGene: 23668 GeneCards: LRRIQ3 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Gene
Locus
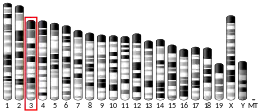
LRRIQ3 is found on the minus strand of the end of the short arm of human chromosome 1 at 1p31.1.[7]
mRNA
Expression
LRRIQ3 is expressed as 2 primary isoforms, which produce proteins of length 624 amino acids and 464 amino acids respectively.[7] It is expressed at low levels in human and brown rat tissues,[8][9] with highest expression levels in testes tissue. There are relatively high expression levels in T cells, the epididymis, the kidney, and a number of glands.[10]
Protein
General Characteristics and Compositional Features
Human protein LRRIQ3 Isoform 1 consists of 624 amino acids, and has a molecular weight of 73.7 kDa. The isoelectric point of LRRIQ3 is 9.73, which suggests that LRRIQ3 is basic at normal physiological pH (~7.4).[11] Additionally, there is strong evidence that human LRRIQ3 localizes to the plasma membrane from antibody staining.[12] LRRIQ3 is rich in lysine residues, with a total of 82 lysines. It is also slightly low on glycines.[13]
Domains and Motifs
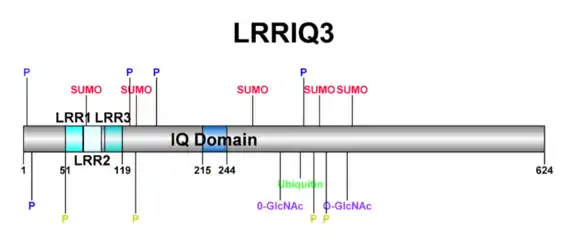
In total, there are 4 conserved domains within LRRIQ3: 3 leucine-rich repeats and 1 IQ calmodulin-binding motif.[13] Leucine-rich repeats are typically involved in protein-protein interactions, and form a characteristic α/β horseshoe fold.[14][15] An IQ motif provides a binding site for calmodulin (CaM) or CaM-like proteins.[16]
Secondary and Tertiary Structure
LRRIQ3 is predicted to be mostly alpha-helical in structure, including a long alpha-helical C-terminal domain. It is also predicted to function as a monomer.[17][18][19][20]
Post-translational Modifications
LRRIQ3 is predicted to undergo many post-translational modifications. These include O-GlcNAcylation, SUMOylation, ubiquitination, and phosphorylation.[22][23] LRRIQ3 is predicted to have 4 well conserved SUMOylation sites and 1 well conserved ubiquitination site.[22] A representation of these post-translational modifications is shown in the figure below.
Protein Interactions
There is evidence that LRRIQ3 interacts with a number of proteins from two-hybrid assays and affinity chromatography. The proteins LRRIQ3 interact with include LYN, NCK2, GNB4, and ABL1.[25][26] These proteins are associated with cell signalling, cytoskeletal reorganization, and cell differentiation, as well as others.[27][28][29][30]
Homology and evolution
Paralogs and Orthologs
No paralogs exists for LRRIQ3 in humans.[6] However, there are a number of orthologs, as reported by BLAST, some of which are listed below.[31] The number of years since divergence from the human protein, listed in "million of years ago (MYA)" below, were calculated using TimeTree.[32]
| Genus and Species | Common Name | Divergence from Human Lineage (MYA) | Accession Number | Sequence length (aa) | Sequence Identity to Human Protein | Sequence Similarity to Human Protein |
|---|---|---|---|---|---|---|
| Gorilla gorilla gorilla | Gorilla | 9.06 | XP_004026030.1 | 624 | 97% | 98% |
| Macaca mulatta | Rhesus monkey | 29.44 | XP_001097148.2 | 623 | 93% | 95% |
| Ursus maritimus | Polar bear | 96 | XP_008689049.1 | 625 | 76% | 87% |
| Felis catus | Domestic cat | 96 | XP_003990274.1 | 625 | 74% | 86% |
| Camelus ferus | Bactrian camel | 96 | XP_006178380.1 | 618 | 73% | 84% |
| Oryctolagus cuniculus | European rabbit | 90 | XP_002715603.1 | 622 | 71% | 83% |
| Bison bison bison | American bison | 96 | XP_010847739.1 | 625 | 70% | 82% |
| Trichechus manatus latirostris | Manatee | 105 | XP_004369192.1 | 623 | 70% | 82% |
| Loxodonta africana | African elephant | 105 | XP_003411181.1 | 625 | 68% | 80% |
| Condylura cristata | Star-nosed mole | 96 | XP_004679575.1 | 627 | 67% | 80% |
| Eptesicus fuscus | Big brown bat | 96 | XP_008137759.1 | 621 | 66% | 80% |
| Myotis davidii | Vesper bat | 96 | XP_006775977.1 | 618 | 65% | 79% |
| Rattus norvegicus | Norway rat | 90 | NP_001019478.1 | 633 | 62% | 77% |
| Mus Musculus | House mouse | 90 | NP_083214.2 | 633 | 63% | 76% |
| Sorex araneus | Common shrew | 96 | XP_004603704.1 | 612 | 55% | 73% |
| Chrysemys picta bellii | Painted turtle | 312 | XP_005285573.1 | 624 | 40% | 56% |
| Pogona vitticeps | Bearded dragon | 312 | XP_020650341.1 | 651 | 35% | 54% |
| Apteryx australis mantelli | Brown kiwi | 312 | XP_013800580.1 | 664 | 35% | 54% |
| Struthio camelus australis | Southern Ostrich | 312 | XP_009685099.1 | 628 | 34% | 51% |
Clinical significance
LRRIQ3 is linked to a number of cancers. RNA-seq experiments have shown that LRRIQ3 is severely down-regulated (Log2-fold changes between -3.4 and -4.2) in a number of disease states, including pancreatic cancer, colorectal cancer, and breast cancer.[33][34][35]
References
- GRCh38: Ensembl release 89: ENSG00000162620 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000028182 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "LRRIQ3 Gene - GeneCards".
- "AceView entry on LRRIQ3".
- "LRRIQ3 leucine rich repeats and IQ motif containing 3 [Homo sapiens (human)] - Gene - NCBI". www.ncbi.nlm.nih.gov. Retrieved 2018-04-30.
- "Lrriq3 protein abundance in PaxDb". pax-db.org. Retrieved 2018-04-30.
- "LRRIQ3 protein abundance in PaxDb". pax-db.org. Retrieved 2018-04-30.
- "GDS3834 / 3169". www.ncbi.nlm.nih.gov. Retrieved 2018-05-06.
- "ExPASy - Compute pI/Mw tool". web.expasy.org. Retrieved 2018-04-30.
- "Cell atlas - LRRIQ3 - The Human Protein Atlas". www.proteinatlas.org. Retrieved 2018-04-30.
- EMBL-EBI. "SAPS < Sequence Statistics < EMBL-EBI". www.ebi.ac.uk. Retrieved 2018-04-30.
- Kobe B, Deisenhofer J (October 1994). "The leucine-rich repeat: a versatile binding motif". Trends Biochem. Sci. 19 (10): 415–21. doi:10.1016/0968-0004(94)90090-6. ISSN 0968-0004. PMID 7817399.
- Enkhbayar P, Kamiya M, Osaki M, Matsumoto T, Matsushima N (February 2004). "Structural principles of leucine-rich repeat (LRR) proteins". Proteins. 54 (3): 394–403. doi:10.1002/prot.10605. ISSN 1097-0134. PMID 14747988. S2CID 19951452.
- Rhoads AR, Friedberg F (April 1997). "Sequence motifs for calmodulin recognition". FASEB J. 11 (5): 331–40. doi:10.1096/fasebj.11.5.9141499. ISSN 0892-6638. PMID 9141499. S2CID 1877645.
- Rost B (2001). "Review: protein secondary structure prediction continues to rise". J. Struct. Biol. 134 (2–3): 204–18. CiteSeerX 10.1.1.8.8169. doi:10.1006/jsbi.2001.4336. ISSN 1047-8477. PMID 11551180.
- Ouali M, King RD (June 2000). "Cascaded multiple classifiers for secondary structure prediction". Protein Sci. 9 (6): 1162–76. doi:10.1110/ps.9.6.1162. ISSN 0961-8368. PMC 2144653. PMID 10892809.
- Cuff JA, Barton GJ (August 2000). "Application of multiple sequence alignment profiles to improve protein secondary structure prediction". Proteins. 40 (3): 502–11. doi:10.1002/1097-0134(20000815)40:3<502::AID-PROT170>3.0.CO;2-Q. ISSN 0887-3585. PMID 10861942. S2CID 855816.
- Jones DT (September 1999). "Protein secondary structure prediction based on position-specific scoring matrices". J. Mol. Biol. 292 (2): 195–202. doi:10.1006/jmbi.1999.3091. ISSN 0022-2836. PMID 10493868. S2CID 15506630.
- Yang J, Yan R, Roy A, Xu D, Poisson J, Zhang Y (January 2015). "The I-TASSER Suite: protein structure and function prediction". Nat. Methods. 12 (1): 7–8. doi:10.1038/nmeth.3213. ISSN 1548-7091. PMC 4428668. PMID 25549265.
- Pagni M, Ioannidis V, Cerutti L, Zahn-Zabal M, Jongeneel CV, Falquet L (July 2004). "MyHits: a new interactive resource for protein annotation and domain identification". Nucleic Acids Res. 32 (Web Server issue): W332–5. doi:10.1093/nar/gkh479. ISSN 0305-1048. PMC 441617. PMID 15215405.
- de Castro E, Sigrist CJ, Gattiker A, Bulliard V, Langendijk-Genevaux PS, Gasteiger E, Bairoch A, Hulo N (July 2006). "ScanProsite: detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins". Nucleic Acids Res. 34 (Web Server issue): W362–5. doi:10.1093/nar/gkl124. ISSN 1362-4962. PMC 1538847. PMID 16845026.
- Ren J, Wen L, Gao X, Jin C, Xue Y, Yao X (February 2009). "DOG 1.0: illustrator of protein domain structures". Cell Res. 19 (2): 271–3. doi:10.1038/cr.2009.6. ISSN 1001-0602. PMID 19153597.
- "Results - mentha: the interactome browser". mentha.uniroma2.it. Retrieved 2018-04-30.
- "LRRIQ3 - Leucine-rich repeat and IQ domain-containing protein 3 - Homo sapiens (Human) - LRRIQ3 gene & protein". www.uniprot.org. Retrieved 2018-04-30.
- Harder KW, Parsons LM, Armes J, Evans N, Kountouri N, Clark R, Quilici C, Grail D, Hodgson GS, Dunn AR, Hibbs ML (October 2001). "Gain- and loss-of-function Lyn mutant mice define a critical inhibitory role for Lyn in the myeloid lineage". Immunity. 15 (4): 603–15. doi:10.1016/s1074-7613(01)00208-4. ISSN 1074-7613. PMID 11672542.
- Downes GB, Gautam N (December 1999). "The G protein subunit gene families". Genomics. 62 (3): 544–52. doi:10.1006/geno.1999.5992. ISSN 0888-7543. PMID 10644457.
- Tu Y, Li F, Wu C (December 1998). "Nck-2, a novel Src homology2/3-containing adaptor protein that interacts with the LIM-only protein PINCH and components of growth factor receptor kinase-signaling pathways". Mol. Biol. Cell. 9 (12): 3367–82. doi:10.1091/mbc.9.12.3367. ISSN 1059-1524. PMC 25640. PMID 9843575.
- Era T (July 2002). "Bcr-Abl is a "molecular switch" for the decision for growth and differentiation in hematopoietic stem cells". Int. J. Hematol. 76 (1): 35–43. doi:10.1007/BF02982716. PMID 12138893. S2CID 10269867.
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (October 1990). "Basic local alignment search tool". J. Mol. Biol. 215 (3): 403–10. doi:10.1016/S0022-2836(05)80360-2. ISSN 0022-2836. PMID 2231712. S2CID 14441902.
- "TimeTree :: The Timescale of Life". www.timetree.org. Retrieved 2018-05-06.
- "Tissue expression of LRRIQ3 - Summary - The Human Protein Atlas". www.proteinatlas.org. Retrieved 2018-05-06.
- github.com/gxa/atlas/graphs/contributors, EMBL-EBI Expression Atlas development team. "Search results < Expression Atlas < EMBL-EBI". www.ebi.ac.uk. Retrieved 2018-04-30.
{{cite web}}:|last=has generic name (help) - github.com/gxa/atlas/graphs/contributors, EMBL-EBI Expression Atlas development team. "Experiment < Expression Atlas < EMBL-EBI". www.ebi.ac.uk. Retrieved 2018-05-06.
{{cite web}}:|last=has generic name (help)