LSMEM1
Leucine-Rich Single-Pass Membrane Protein 1 (LSMEM1) is a protein that, in humans, is encoded by the LSMEM1 gene.
Gene

In humans, LSMEM1 is located on chromosome 7q31.1.[1][2] LSMEM1 neighbors the gene IFRD1 in humans. Aliases for LSMEM1 include C7orf53, chromosome 7 open reading frame 53, and FLJ39575.[3] The human mRNA is 1686 base pairs long and the gene contains 5 exons.[4] The human mRNA also has a 5' UTR and a 3' UTR. The 5' UTR goes from mRNA position 1 to 341, and the 3' UTR goes from mRNA position 738 to 1686.
Protein
The protein LSMEM1 encodes in humans is 131 amino acids long[5] and has a molecular weight ranging from 14.2 to 14.5 kDal.[6] Its isoelectric point in humans is about 5, making it slightly acidic. LSMEM1 is an integral membrane protein and a transmembrane protein Besides its transmembrane segment, it is mainly made up of coils and relatively few beta strands. As its name implies, LSMEM1 only has one transmembrane segment. The predicted post-translational modifications to the human LSMEM1 protein are glycation[7] and phosphorylation.[8] There is no predicted signal peptide for the human protein.[9]
Homology/Evolution
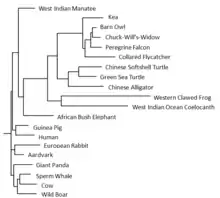
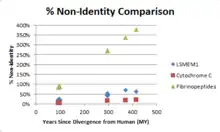
There are no known paralogs in humans for LSMEM1.[10] Orthologous proteins exist mainly in mammals, birds, and reptiles. There are also more distant orthologs in amphibians and sarcopterygii. LSMEM1 does not show up in invertebrates, fungi, or prokaryotes. LSMEM1 also contains a conserved domain of unknown function DUF4577.[3] When the human protein encoded by LSMEM1 is compared to a known quickly evolving protein (fibrinopeptides) and a known slowly evolving protein (cytochrome c), LSMEM1 appears to be slowly evolving.
Expression
In humans, LSMEM1 is very highly expressed in skeletal muscle.[11] In humans, LSMEM1 also shows high expression in nerve tissue, moderate expression in the uterus, testis, bone marrow, heart, and intestines, and low expression in the brain and pancreas.[12] It also shows expression in both the fetal and adult stages of life in humans.
LSMEM1 is predicted to have a 615 base pair promoter in humans just upstream of its transcriptional start site.[13]
Interacting Proteins
Some transcription factors that are predicted to bind to the promoter of LSMEM1 in humans are: SRF, EVI1, SOX, ETS, LTSM, and TALE transcription factors.[13] Many of the transcription factors thought to bind to the LSMEM1 promoter in humans have activity in biological processes like cell differentiation, cell cycle, cell growth, and development.
There are 3 main proteins thought to interact with the human protein encoded by the gene LSMEM1 that were determined via two-hybrid screening (LSMEM2 and MAL) and reconstituted complex (APP) experiments. A reconstituted complex experiment detects interactions between purified proteins in vitro. The 3 proteins thought to interact with LSMEM1 are: MAL, APP, and LSMEM2.[14] All three of these interacting proteins are integral membrane proteins, just as LSMEM1 is.
Clinical Significance
LSMEM1 expression levels in humans are greatly decreased in septic skeletal muscle.[15] For humans, the LSMEM1 protein has 17 SNP missense mutations within its coding sequence.[16] The LSMEM1 protein has been shown to be prevalent in the serum of patients with Parkinson's disease and that it binds to diagnostic biomarkers of Parkinson's Disease as a protein antigen.[17]
References
- Scherer, S. W. (10 April 2003). "Human Chromosome 7: DNA Sequence and Biology". Science. 300 (5620): 767–772. Bibcode:2003Sci...300..767S. doi:10.1126/science.1083423. PMC 2882961. PMID 12690205.
- "LSMEM1 leucine-rich single-pass membrane protein 1 [ Homo sapiens (human) ]". NCBI. Retrieved 22 April 2015.
- "Gene: LSMEM1". Ensembl. Retrieved 30 April 2015.
- "Homo sapiens leucine-rich single-pass membrane protein 1 (LSMEM1), transcript variant 1, mRNA". National Center for Biotechnology Information Nucleotide. Retrieved 8 May 2015.
- "Chromosome 7 open reading frame 53 [Homo sapiens]". NCBI. Retrieved 22 April 2015.
- "Biology Workbench". San Diego Supercomputer Center. Retrieved 30 April 2015.
- Gupta, R.; Jung, E.; Brunak, S. "Prediction of N-glycosylation sites in human proteins". ExPASy. Retrieved 30 April 2015.
- Blom, N.; Gammeltoft, S.; Brunak, S. (December 1999). "Sequence- and structure-based prediction of eukaryotic protein phosphorylation sites". Journal of Molecular Biology. 294 (5): 1351–62. doi:10.1006/jmbi.1999.3310. PMID 10600390. Retrieved 30 April 2015.
- Petersen, TN; Brunak, S; von Heijne, G; Nielsen, H (29 September 2011). "SignalP 4.0: discriminating signal peptides from transmembrane regions". Nature Methods. 8 (10): 785–6. doi:10.1038/nmeth.1701. PMID 21959131.
- "Basic Local Alignment Search Tool". National Center for Biotechnology Information. Retrieved 30 April 2015.
- Liu, D.; Sartor, M. A.; Nader, G. A.; Pistilli, E. E.; Tanton, L.; Lilly, C.; Gutmann, L.; IglayReger, H. B.; Visich, P. S.; Hoffman, E. P.; Gordon, P. M. (15 February 2013). "Microarray Analysis Reveals Novel Features of the Muscle Aging Process in Men and Women". The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 68 (9): 1035–1044. doi:10.1093/gerona/glt015. PMC 3826860. PMID 23418191.
- "EST Profile". National Center for Biotechnology Information. Retrieved 6 May 2015.
- "ElDorado". Genomatix. Retrieved 5 May 2015.
- "LSMEM1". Mentha The Interactome Browser. Retrieved 1 May 2015.
- Fredriksson, Katarina; Tjäder, Inga; Keller, Pernille; Petrovic, Natasa; Ahlman, Bo; Schéele, Camilla; Wernerman, Jan; Timmons, James A.; Rooyackers, Olav; Bozza, Patricia (10 November 2008). "Dysregulation of Mitochondrial Dynamics and the Muscle Transcriptome in ICU Patients Suffering from Sepsis Induced Multiple Organ Failure". PLOS ONE. 3 (11): e3686. Bibcode:2008PLoSO...3.3686F. doi:10.1371/journal.pone.0003686. PMC 2579334. PMID 18997871.
- "dbSNP Short Genetic Variations". National Center for Biotechnology Information. Retrieved 4 May 2015.
- Nagele, Robert. "Diagnostic Biomarker Profiles for the Detection and Diagnosis of Parkinsons Disease". FPO. Retrieved 7 May 2015.