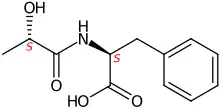
Lac-Phe
Lactoylphenylalanine, or Lac-Phe, is a metabolite generated by intense exercise.[1][2][3] In mice, high levels of Lac-Phe in the blood cause a decrease of food intake.[1] In mammals it is created from (S)-lactate and L-phenylalanine by the cytosol nonspecific dipeptidase (CNDP2) protein.[4] It is classified as N-acyl-alpha-amino acid and pseudodipeptide.[5]
 | |
| Names | |
|---|---|
| IUPAC name
(2S)-2-[[(2S)-2-hydroxypropanoyl]amino]-3-phenylpropanoic acid | |
| Other names
N-[(S)-lactoyl]-L-phenylalaninate N-Lactoyl-phenylalanine N-Lactoylphenylalanine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C12H15NO4 | |
| Molar mass | 237.255 g·mol−1 |
| Related compounds | |
Related N-acyl-alpha-amino acids |
N-Acetylaspartic acid N-acetylcysteine N-Acetylglutamic acid N-Acetylglutamine N-Acetylleucine N-formylmethionine |
Related compounds |
Lactamide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
It has also been reported that as an additive N-L-lactoyl phenylalanine improves the taste of food, conferring an umami flavor. It is found naturally in significant amounts in some traditional Chinese fermented foods such as preserved pickles and soy sauce.[6] Oral intake of Lac-Phe does not have anti-obesity effects in mice.[1]
See also
- Acyl group
- Lactoyl, the acyl group derived from lactic acid
- Alpha-amino acid
- Dipeptide
- Dipeptidase
References
- Li VL, He Y, Contrepois K, Liu H, Kim JT, Wiggenhorn AL, et al. (June 2022). "An exercise-inducible metabolite that suppresses feeding and obesity". Nature. 606 (7915): 785–790. doi:10.1038/s41586-022-04828-5. PMC 9767481. PMID 35705806. S2CID 249710767.
- Wong C (15 June 2022). "Appetite-suppressing molecule helps obese mice lose weight". New Scientist. Retrieved 18 June 2022.
- Reynolds G (15 June 2022). "Why Does a Hard Workout Make You Less Hungry?". The New York Times. Retrieved 18 June 2022.
- Jansen RS, Addie R, Merkx R, Fish A, Mahakena S, Bleijerveld OB, et al. (May 2015). "N-lactoyl-amino acids are ubiquitous metabolites that originate from CNDP2-mediated reverse proteolysis of lactate and amino acids". Proceedings of the National Academy of Sciences of the United States of America. 112 (21): 6601–6606. Bibcode:2015PNAS..112.6601J. doi:10.1073/pnas.1424638112. PMC 4450436. PMID 25964343.
- "Metabocard for N-Lactoylphenylalanine". The Human Metabolome Database (HMDB). The Metabolomics Innovation Centre (TIMC). HMDB0062175.
- Wu J, Gao J, Lin J, Cui C, Li L, He S, Brennan C (May 2022). "Preparation and Taste Characteristics of Kokumi N-Lactoyl Phenylalanine in the Presence of Phenylalanine and Lactate". Journal of Agricultural and Food Chemistry. 70 (17): 5396–5407. doi:10.1021/acs.jafc.2c00530. PMID 35452224. S2CID 248345604.
External links
- Lowe, Derek. "A Metabolite of Exercise". In the pipeline-science.org.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.