Lactobionic acid
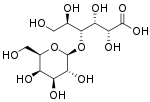
Lactobionic acid (4-O-β-galactopyranosyl-D-gluconic acid) is a sugar acid. It is a disaccharide formed from gluconic acid and galactose. It can be formed by oxidation of lactose. The carboxylate anion of lactobionic acid is known as lactobionate.
 | |
| Names | |
|---|---|
| IUPAC name
(2R,3R,4R)-2,3,5,6-Tetrahydroxy-4-[ [(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)-2-tetrahydropyranyl]oxy]hexanoic acid | |
| Other names
Galactosylgluconic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.002.309 |
| E number | E399 (antioxidants, ...) |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C12H22O12 | |
| Molar mass | 358.296 g·mol−1 |
| Appearance | Syrup[1] |
| Freely soluble[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
As an acid, lactobionic acid can form salts with mineral cations such as calcium, potassium, sodium and zinc. Calcium lactobionate is a food additive used as a stabilizer. Potassium lactobionate is added to organ preservation solutions such as Viaspan or CoStorSol to provide osmotic support and prevent cell swelling. Mineral salts of lactobionic acid are also used for mineral supplementation.
Lactobionic acid is also used in the cosmetics industry as an antioxidant[2] and in the pharmaceutical industry as an excipient for formulation. For example, the antibiotic erythromycin is used as the salt erythromycin lactobionate when intravenously delivered.
References
- Merck Index, 11th Edition, 5219
- Tasic-Kostov, M.; Pavlovic, D.; Lukic, M.; Jaksic, I.; Arsic, I.; Savic, S. (2012-10-12). "Lactobionic acid as antioxidant and moisturizing active in alkyl polyglucoside-based topical emulsions: the colloidal structure, stability and efficacy evaluation". International Journal of Cosmetic Science. 34 (5): 424–434. doi:10.1111/j.1468-2494.2012.00732.x. ISSN 1468-2494. PMID 22691034. S2CID 2854711.