LECT2 amyloidosis
LECT2 Amyloidosis (ALECT2) is a form of amyloidosis caused by the LECT2 protein. It was found to be the third most common (~3% of total) cause of amyloidosis in a set of more than 4,000 individuals studied at the Mayo Clinic; the first and second most common forms the disorder were AL amyloidosis and AA amyloidosis, respectively. Amyloidosis is a disorder in which the abnormal deposition of a protein in organs and/or tissues gradually leads to organ failure and/or tissue injury.[1][2][3]
| LECT2 amyloidosis | |
|---|---|
 | |
| Example of amyloid deposits under Congo Red staining |
Although more than 30 different proteins can cause amyloidosis, the disorder caused by LECT2 is distinctive in three ways. First, it has an unusually high incidence in certain ethnic populations. Second, it is a systemic form of amyloidosis (i.e. amyloid deposited in multiple organs), as opposed to a localized form (amyloid deposits limited to a single organ) but nonetheless injures the kidney without or rarely injuring the other organs in which it is deposited. Third, LECT2 amyloidosis is diagnosed almost exclusively in elderly individuals.[2]
Given its relatively recent discovery, exceptionally strong ethnic bias, limitation to causing kidney disease, and restriction to elderly individuals, LECT2 amyloidosis appears at present to be an under-recognized cause of chronic kidney disease particularly in the ethnic groups that exhibit a high incidence of the disorder.[4]
Presentation
Most individuals diagnosed with LECT2 amyloidosis in the United States (88%) are of Mexican descent and reside in Southwest region of the United States (New Mexico, Arizona, far Western Texas). Other groups with higher incidence rates of the disorder include First Nation Peoples in Canada, Punjabis, South Asians, Sudanese, Native Americans, and Egyptians. In Egyptians, for example, LECT2 is second most common cause of renal amyloidosis, accounting for nearly 31% of all cases.[5][6][7][8][9]
LECT2 amyloidosis (ALECT2) is generally diagnosed in individuals between the ages 40 and 90, with a mean age of 67 years old. The disorder commonly presents with renal disease that in general is advanced or at an end stage. Associated signs and symptoms of their renal disease may include fatigue, dehydration, blood in urine, and/or other evidence for the presence of the nephrotic syndrome or renal failure. Further studies may find that these individuals have histological or other evidence of LECT2 amyloid deposition in the liver, lung, spleen, kidney, and/or adrenal glands but nonetheless they rarely show any symptoms or signs attributable to dysfunction in these organs. Unlike many other forms of systemic amyloidosis, LECT2 deposition has not been reported to be deposited in the myocardium or brain of affected individuals. Thus, LECT2 amyloidosis, while classified as a form of systemic amyloidosis, almost exclusively manifests clinically as renal amyloidosis.[10][6][7][11][12][13][14] No familial link has been found in the disorder although there have been several cases described among siblings.[10]
Cause
Gene
The human LECT2 gene, LECT2, is located on the long, i.e, "q", arm of chromosome 5 at position q31.1 (notated as 5q31.1). This location is close to several immune modulating genes including interleukins 3, 5, and 9 and granulocyte-macrophage colony stimulating factor. LECT2 is conserved in zebrafish, chicken, rat, mouse, cow. dog, Rhesus monkey, and chimpanzee. Human LECT2 is composed of 4 exons, 3 introns, and ~8,000 base pairs. The gene has numerous single nucleotide variants as well as other variations, two of which (see Pathophysiology section) have been associated with human disease. Human LECT2 has several different transcriptional initiation sights and codes for a mRNA composed of 1,000 to 1,300 ribonucleotides. mRNA for LECT2 is highly expressed in liver tissue and expressed at far lower levels in a wide range of other tssues.[2][15]
Protein
The LECT2 protein consists of 133`amino acids Its structure is similar to that of the M23 family of metalloendopeptidases. Unlike this family of peptidases, however, LECT2 has not been found to possess enzymatic activity and does not appear to share any functions with M23 metalloendopeptidases.[2][16] It is widely expressed in vascular tissues, smooth muscle cells, adipocytes, cerebral neurons, apical squamous epithelia, parathyroid tissues, the epithelial cells of sweat and sebaceous glands, Hassall bodies, and monocytes. The liver hepatocyte is considered to be the source of the LECT2 circulating in blood. However, its expression in these cells is extremely low or undetectable even though these cells express very high levels of LECT2 mRNA. This implies that hepatocytes secrete LECT2 almost immediately after they make it. Using very sensitive methods, LECT2 protein can also be detected at low levels in the endothelial cells of hepatic arteries and veins including central veins. Several cell types or tissues, e.g. osteoblasts, chondrocytes, cardiac tissue, gastrointestinal smooth muscle cells, and epithelial cells of some tissues normally do not express LECT2 but do so under a variety of disease conditions.[2]
Pathophysiology
LECT2 as a hepatokine, a substance made and released into the circulation by liver hepatocyte cells that acts as a hormone or signaling agent to regulate the function of other cells.[2] While the pathogenesis of LECT2 amyloidosis is unclear, the intact LECT2 protein may have a tendency to fold abnormally thereby forming non-soluble fibrils that are deposited in tissues. It has been suggested that individuals with the disease have an increase in LECT2 production and/or a decrease in LECT2 catabolism (i.e. breakdown) which may increase its tendency to deposit in tissues. On the other hand, there are genetic variations which appear to cause the deposition of LECT2 in tissues. Studies to date have failed to obtain evidence for LECT2 gene mutations in the disorder but most cases examined in the United States are associated with a particular homozygous single nucleotide polymorphism (i.e. SNP) in the LECT2 gene. This SNP occurs in exon 3 at codon 58 of the gene, contains a guanine rather than adenine nucleotide at this site, and consequently codes for the amino acid valine rather than isoleucine. Although not yet proven to occur in vivo, the Val58Ile variant of LECT2 may have a propensity to fold abnormally, form insoluble fibrils, and therefore deposits in tissues. The Val58Ile LECT2 variant is common in Hispanics and appears to be the cause of their high incidence of LECT2 amyloidosis. However, not all homozygous Hispanic carriers of the variant ever exhibit LECT2 amyloidosis. This suggests that another factor(s) besides the variant Val58Ile protein's structure is involved in its organ/tissue deposition.[2]
A second SNP which is also commonly found in Mexicans occurs at codon 172 of the LECT2 gene. This variant is homozygous for a G nucleotide at this codon position and is associated with an increased incidence of LECT2 amyloidosis. A reason for this association has not yet been proposed.[2][17]
It has been found repeatedly that the mere presence of LECT2 amyloid tissue deposits does not necessarily indicate the presence of LECT2 amyloidosis disease. For example, autopsy studies find that up to 3.1% of Hispanics have these deposits in their kidneys but no history of signs or symptoms that could be attributed to LECT2 amyloidosis. This finding suggests that the LECT2 amyloidosis and its ethnic bias reflect multiple poorly understood factors.[2]
Diagnosis
LECT2 amyloidosis is diagnosed by a kidney biopsy which reveals two key findings: a) histological evidence of Congo red staining material deposited in the interstitial, mesangial, glomerular, and/or vascular areas of the kidney and b) the identification of these deposits as containing mainly LECT2 as identified by proteomics methodologies. Kidney biopsy shows the presence of LECT2-based amyloid predominantly in the renal cortex interstitium, glomeruli, and arterioles.[17][1] LECT2 amyloidosis can be distinguished from AL amyloidosis, the most common form of amyloidosis (~85% of total cases), by testing their blood for the presence of high levels of a clonal immunoglobulin light chain. If the patient tests negative for this light chain, positive Congo Red staining of the kidney biopsy strongly suggests LECT2 amyloidosis.[1][11]
Treatment
There has too little experience on the treatment of LECT2 amyloidosis to establish recommendations other than offering methods to support kidney function and dialysis. Nonetheless, it is important to accurately diagnose ALECT2-based amyloid disease in order to avoid treatment for other forms of amyloidosis.[1][17]
Prognosis
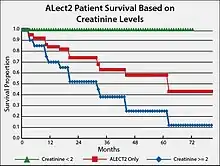
Based on studies conducted in the United States, the prognosis for individuals with ALECT2 is guarded, particularly because they are elderly and their kidney disease is usually well-advanced at the time of presentation.[17] End-stage renal disease develops in 1 out of 3 patients and has a median renal survival of 62 months.[10] A suggested prognostic tool is to track creatinine levels in ALECT2 patients. The attached Figure gives survival plotss for individuals with LECT2 renal amyloidosis and serum creatinine levels less than 2 mg/100 ml versus 2 mg/100 ml or greater than 2 mg/100 ml. The results show that afflicted individuals with lower creatinine levels have a ~four-fold higher survival rate.
See also
References
- Holanda DG, Acharya VK, Dogan A, Racusen LC and Atta MG. Atypical Presentation of Atypical Amyloid. Nephrology dialysis transplantation.2011; 26(1):373-6.
- Picken, M. (Aug 2014). "Alect2 amyloidosis: Primum non nocere (first, do no harm)". Kidney Int. 86 (2): 229–232. doi:10.1038/ki.2014.45. PMID 25079018.
- Slowik V, Apte U (2017). "Leukocyte Cell-Derived Chemotaxin-2: It's [sic] Role in Pathophysiology and Future in Clinical Medicine". Clinical and Translational Science. 10 (4): 249–259. doi:10.1111/cts.12469. PMC 5504477. PMID 28466965.
- Meex RC, Watt MJ (2017). "Hepatokines: linking nonalcoholic fatty liver disease and insulin resistance". Nature Reviews. Endocrinology. 13 (9): 509–520. doi:10.1038/nrendo.2017.56. PMID 28621339.
- Larsen CP, Beggs ML, Wilson JD, Lathrop SL (2016). "Prevalence and organ distribution of leukocyte chemotactic factor 2 amyloidosis (ALECT2) among decedents in New Mexico". Amyloid. 23 (2): 119–23. doi:10.3109/13506129.2016.1145110. PMC 4898138. PMID 26912093.
- Dogan, A.; Theis, J.D.; Vrana, J.A.; Jimenez-epeda, V.H.; Lacy, M.Q.; Leung, N. (2010). "Clinical and pathological phenotype of leukocyte cell-derived chemotaxin-2 (LECT2) amyloidosis (ALECT2)". Amyloid: 69–70.
- Hutton, H.L.; DeMarco, M.L.; Magil, A.B.; Taylor, P. (2014). "Renal leukocyte chemotactic factor 2 (LECT2) amyloidosis in first nations people in northern british columbia, canada: A report of 4 cases". Am J Kidney Dis. 64 (5): 790–792. doi:10.1053/j.ajkd.2014.06.017. PMID 25064673.
- Murphy, C. L.; Wang, S.; Kestler, D.; Larsen, C.; Benson, D.; Weiss, D. T. (2010). "Leukocyte chemotactic factor 2 (LECT2)-associated renal amyloidosis: A case series". Am J Kidney Dis. 56 (6): 1100–1107. doi:10.1053/j.ajkd.2010.08.013. PMC 2991509. PMID 20951486.
- Sethi S, Theis JD (2017). "Pathology and diagnosis of renal non-AL amyloidosis". Journal of Nephrology. 31 (3): 343–350. doi:10.1007/s40620-017-0426-6. PMID 28828707.
- Larsen CP, Ismail W, Kurtin PJ, Vrana JA, Dasari S, Nasr SH (2016). "Leukocyte chemotactic factor 2 amyloidosis (ALECT2) is a common form of renal amyloidosis among Egyptians". Modern Pathology. 29 (4): 416–20. doi:10.1038/modpathol.2016.29. PMC 5411489. PMID 26867784.
- Said SM, Sethi S, Valeri AM, Chang A, Nast CC, Krahl L, Molloy P, Barry M, Fidler ME, Cornell LD, Leung N, Vrana JA, Theis JD, Dogan A, Nasr SH (2014). "Characterization and outcomes of renal leukocyte chemotactic factor 2-associated amyloidosis". Kidney International. 86 (2): 370–7. doi:10.1038/ki.2013.558. PMID 24451324.
- Dogan A (2017). "Amyloidosis: Insights from Proteomics". Annual Review of Pathology. 12: 277–304. doi:10.1146/annurev-pathol-052016-100200. PMID 27959636.
- Benson, M. D.; James, S.; Scott, K.; Liepnieks, J. J.; Kluve-Beckerman, B. (April 2008). "Leukocyte chemotactic factor 2: A novel renal amyloid protein". Kidney Int. 74 (2): 218–222. doi:10.1038/ki.2008.152. PMID 18449172.
- Holanda, D.G.; Acharya, V.K.; Dogan, A.; Racusen, L.C.; Atta, M.G. (Oct 2010). "Atypical presentation of atypical amyloid". Nephrol Dial Transplant. 26 (1): 373–376. doi:10.1093/ndt/gfq638. PMID 20940371.
- Larsen, C. P.; Walker, P.D.; Weiss, D. T.; Solomon, A. (February 2010). "Prevalence and morphology of leukocyte chemotactic factor 2-associated amyloid in renal biopsies". Kidney Int. 77 (9): 816–819. doi:10.1038/ki.2010.9. PMID 20182418.
- "Entrez Gene: LECT2 leukocyte cell-derived chemotaxin 2".
- Zheng H, Miyakawa T, Sawano Y, Asano A, Okumura A, Yamagoe S, Tanokura M (2016). "Crystal Structure of Human Leukocyte Cell-derived Chemotaxin 2 (LECT2) Reveals a Mechanistic Basis of Functional Evolution in a Mammalian Protein with an M23 Metalloendopeptidase Fold". The Journal of Biological Chemistry. 291 (33): 17133–42. doi:10.1074/jbc.M116.720375. PMC 5016117. PMID 27334921.
- Larsen CP, Kossmann RJ, Beggs ML, Solomon A, Walker PD (2014). "Clinical, morphologic, and genetic features of renal leukocyte chemotactic factor 2 amyloidosis". Kidney International. 86 (2): 378–82. doi:10.1038/ki.2014.11. PMID 24522497.