Levulinic acid
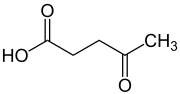
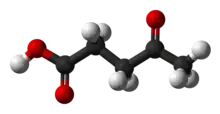
Levulinic acid, or 4-oxopentanoic acid, is an organic compound with the formula CH3C(O)CH2CH2CO2H. It is classified as a keto acid. This white crystalline solid is soluble in water and polar organic solvents. It is derived from degradation of cellulose and is a potential precursor to biofuels,[2] such as ethyl levulinate.[3]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
4-Oxopentanoic acid | |
| Other names
Levulinic acid, β-Acetylpropionic acid, 3-Acetopropionic acid, β-acetylpropionic acid, γ-ketovaleric acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.004.228 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H8O3 | |
| Molar mass | 116.11 g/mol |
| Density | 1.1447 g/cm3 |
| Melting point | 33 to 35 °C (91 to 95 °F; 306 to 308 K) |
| Boiling point | 245 to 246 °C (473 to 475 °F; 518 to 519 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Synthesis
Levulinic acid was first prepared in 1840 by Dutch chemist Gerardus Johannes Mulder by heating fructose with hydrochloric acid.[4] The first commercial production of levulinic acid began as a batchwise process in an autoclave by starch manufacturer A. E. Staley in the 1940s.[5] In 1953 Quaker Oats developed a continuous process for the production of levulinic acid.[6] In 1956 it was identified as a platform chemical with high potential.[7] and in 2004 the US Department of Energy (U.S. DoE) identified levulinic acid as one of the 12 potential platform chemicals in the biorefinery concept.[8]
The synthesis of levulinic acid from hexoses (glucose, fructose) or starch in dilute hydrochloric acid or sulfuric acid.[4][9][10][11] In addition to formic acid further, partly insoluble, by-products are produced. These are deeply colored and their complete removal is a challenge for most technologies.
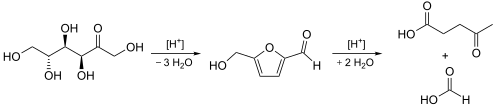
Many concepts for the commercial production of levulinic acid are based on a strong acid technology. The processes are conducted in a continuous manner at high pressures and temperatures. Lignocellulose is an inexpensive starting material. Levulinic acid is separated from the mineral acid catalyst by extraction. Levulinic acid is purified by distillation.[12]
Reactions and applications
Levulinic acid is used as a precursor for pharmaceuticals, plasticizers, and various other additives.[13] The largest application of levulinic acid is its use in the production of aminolevulinic acid, a biodegradable herbicide used in South Asia. Another key application is the use of levulinic acid in cosmetics. Ethyl levulinate, a primary derivative of levulinic acid, is extensively used in fragrances and perfumes. Levulinic acid is a chemical building block or starting material for a wide variety of other compounds[14] including γ-valerolactone and 2-methyl-THF.[8]
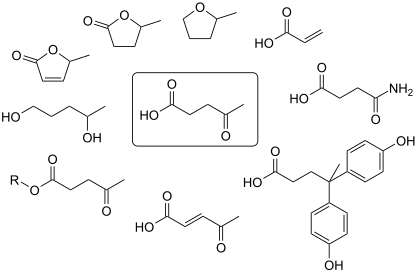
Other occurrence and niche uses
Levulinic acid is used in cigarettes to increase nicotine delivery in smoke and binding of nicotine to neural receptors.[15]
Levulinic acid, in its cyclic alternate structure, was the first pseudoacid to be described as such.
Etymology
The former term “levulose” for fructose gave levulinic acid its name.
References
- The Merck Index, 15th Ed. (2013), p. 1018, Monograph 5526, O'Neil: The Royal Society of Chemistry. Available online at: http://www.rsc.org/Merck-Index/monograph/mono1500005526
- Biorefineries – Industrial Processes and Products. Status Quo and Future Directions. Vol. 1, Edited by Birgit Kamm, Patrick R. Gruber, Michael Kamm. 2006, WILEY-VCH, Weinheim. ISBN 3-527-31027-4
- Leal Silva, Jean Felipe; Grekin, Rebecca; Mariano, Adriano Pinto; Maciel Filho, Rubens (2018). "Making Levulinic Acid and Ethyl Levulinate Economically Viable: A Worldwide Technoeconomic and Environmental Assessment of Possible Routes". Energy Technology. 6 (4): 613–639. doi:10.1002/ente.201700594. ISSN 2194-4296.
- Mulder, G. J. (1840). "Untersuchungen über die Humussubstanzen" [Investigations on humic substances]. J. Prakt. Chem. (in German). 21 (1): 203–240. doi:10.1002/prac.18400210121.
- A. E. Staley, Mfg. Co. (Decatur, Ill.); Levulinic Acid 1942 [C.A. 36, 1612]
- U.S. Patent 2,813,900
- R. H. Leonard, Ind. Eng. Chem. 1331, (1956).
- The Pacific Northwest National Laboratory and The National Renewable Energy Laboratory (Aug 2004). "Volume I-Results of Screening for Potential Candidates from Sugars and Synthesis Gas" (PDF). Top Value Added Chemicals from Biomass. U.S. Department of Energy.
- A. Freiherrn, V. Grote, B. Tollens, "Untersuchungen über Kohlenhydrate. I. Ueber die bei Einwirkung von Schwefelsäure auf Zucker entstehende Säure (Levulinsäure)" Justus Liebigs Annalen der Chemie volume 175, pp. 181-204 (1875). doi:10.1002/jlac.18751750113
- B. F. McKenzie (1941). "Levulinic acid". Organic Syntheses.; Collective Volume, vol. 1, p. 335
- S.L. Suib, New and Future Developments in Catalysis – Catalytic Biomass Conversion, Elsevier, (2013). ISBN 978-0-444-53878-9
- U.S. Patent 5,608,105
- Franz Dietrich Klingler, Wolfgang Ebertz "Oxocarboxylic Acids" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a18_313
- Bozell, Joseph J.; Petersen, Gene R. (2010-04-06). "Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy's "Top 10" revisited". Green Chemistry. 12 (4): 539–554. doi:10.1039/b922014c.
- Doris Cullen et al., A Guide to Deciphering the Internal Codes Used by the Tobacco Industry, Report No. 03-05, Harvard School of Public Health, Division of Public Health Practice, Tobacco Research Program, August 2005, http://legacy.library.ucsf.edu/resources/harvard_monograph.pdf
- Dunstan, Wyndham Rowland (1911). . In Chisholm, Hugh (ed.). Encyclopædia Britannica. Vol. 23 (11th ed.). Cambridge University Press. p. 802.