Lingulodinium polyedra
Lingulodinium polyedra is a species of motile photosynthetic dinoflagellates. L. polyedra are often the cause of red tides in southern California, leading to bioluminescent displays on beaches at night.
| Lingulodinium polyedra | |
|---|---|
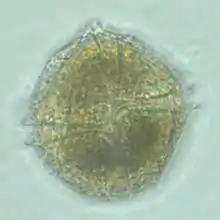 | |
| Ventral view of Lingulodinium polyedrum 900x magnification | |
| Scientific classification | |
| Domain: | Eukaryota |
| Clade: | Diaphoretickes |
| Clade: | SAR |
| Clade: | Alveolata |
| Phylum: | Myzozoa |
| Superclass: | Dinoflagellata |
| Class: | Dinophyceae |
| Order: | Gonyaulacales |
| Family: | Gonyaulacaceae |
| Genus: | Lingulodinium |
| Species: | L. polyedra |
| Binomial name | |
| Lingulodinium polyedra Dodge | |
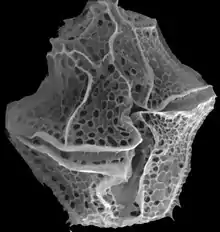

Life cycle
As part of its life cycle, this species produces a resting stage, a dinoflagellate cyst called Lingulodinium machaerophorum (synonym Hystrichosphaeridium machaerophorum). This cyst was first described by Deflandre and Cookson in 1955 from the Miocene of Balcombe Bay, Victoria, Australia as: "Shell globular, subsphaerical or ellipsoidal with a rigid membrane, more brittle than deformable, covered with numerous long, stiff, conical, pointed processes resembling the blade of a dagger. Surface of shell granular or punctate."[1] Its stratigraphic range is the Upper Paleocene of eastern USA [2] and Denmark [3] till Recent.
Organic-walled dinocyst morphology is shown to be controlled by changes in salinity and temperature in some species, more particularly process length variation (processes are sometimes called spines, but that is incorrect because they are not necessarily pointy). This morphological variation is known for Lingulodinium machaerophorum from culture experiments,[4] and study of surface sediments.[5] The morphological variation of process lengths can be applied for the reconstruction of salinity. Process length variation of Lingulodinium machaerophorum has been used to reconstruct Black Sea salinity variation.[6]
Toxicity

Lingulodinium polyedra has been related to production of yessotoxins (YTXs), a group of structurally related polyether toxins, which can accumulate in shellfish and can produce symptoms similar to those produced by Paralytic Shellfish Poisoning (PSP) toxins.[7]
Luminescence
Lingulodinium polyedra are easily visible under 100x magnification (use the 10x or "scanning" objective on most compound microscopes) and their scintillons luminescence in response to surface tension and acidity. Luminescence is under circadian regulation, peaking at night. Because of this obvious rhythms (and also due to the fact that most its activities, physiological and molecular, are rhythmic) L. polyedra has been a model organism for studying clocks in single cells.[8]
References
- Deflandre, G. and Cookson, I.C., 1955. Fossil microplankton from Australian Late Mesozoic and Tertiary sediments. Aust. J. Mar Freshw. Res. 6, 242-313.
- EDWARDS, L.E., GOODMAN, D.K., and WITMER, R.J. 1984 Lower Tertiary (Pamunkey Group) dinoflagellate biostratigraphy, Potomac River area, Virginia and Maryland. In: Frederiksen, N.O., and Krafft, K. (eds.), Cretaceous and Tertiary stratigraphy, paleontology, and structure, southwestern Maryland and northeastern Virginia—Field trip volume and guide book. American Association of Stratigraphic Palynologists Foundation, Dallas, Texas, p. 137–152.
- HEILMANN-CLAUSEN, C. 1985 Dinoflagellate stratigraphy of the uppermost Danian to Ypressian in the Viborg 1 borehole, central Jylland, Denmark. Danmarks Geologiske Undersøgelse, Serie A, 7: 1–69.
- Hallett, R.I., 1999. Consequences of environmental change on the growth and morphology of Lingulodinium polyedrum (Dinophyceae) in culture. Ph.D. thesis. University of Westminster, 109 pp.
- Mertens, K. N., Ribeiro, S. Bouimetarhan, I., Caner, H., Combourieu-Nebout, N. Dale, B., de Vernal, A. Ellegaard, M. Filipova, M., Godhe, A. Grøsfjeld, K. Holzwarth, U. Kotthoff, U. Leroy, S., Londeix, L., Marret, F., Matsuoka, K., Mudie, P., Naudts, L., Peña-manjarrez, J., Persson, A., Popescu, S., Sangiorgi, F., van der Meer, M., Vink, A., Zonneveld, K., Vercauteren, D., Vlassenbroeck, J., Louwye, S., 2009a. Process length variation in cysts of a dinoflagellate, Lingulodinium machaerophorum, in surface sediments investigating its potential as salinity proxy. Marine Micropaleontology 70, 54–69.
- Mertens, K.N., Bradley, L.R., Takano, Y., Mudie, P.J., Marret, F., Aksu, A.E., Hiscott, R.N., Verleye, T.J., Mousing, E.A., Smyrnova, L.L., Bagheri, S., Mansor, M., Pospelova, V. & Matsuoka, K. 2012. Quantitative estimation of Holocene surface salinity variation in the Black Sea using dinoflagellate cyst process length. Quaternary Science Reviews.
- Paz et al. 2008. Yessotoxins, a Group of Marine Polyether Toxins: an Overview. Mar. Drugs 2008, 6, 73-102; DOI: 10.3390/md20080005
- Hastings JW. 2008. The Gonyaulax clock at 50: translational control of circadian expression. Cold Spring Harb Symp Quant Biol. 2007;72:141-4. doi: 10.1101/sqb.2007.72.026