Localized surface plasmon
A localized surface plasmon (LSP) is the result of the confinement of a surface plasmon in a nanoparticle of size comparable to or smaller than the wavelength of light used to excite the plasmon. When a small spherical metallic nanoparticle is irradiated by light, the oscillating electric field causes the conduction electrons to oscillate coherently. When the electron cloud is displaced relative to its original position, a restoring force arises from Coulombic attraction between electrons and nuclei. This force causes the electron cloud to oscillate. The oscillation frequency is determined by the density of electrons, the effective electron mass, and the size and shape of the charge distribution.[1] The LSP has two important effects: electric fields near the particle's surface are greatly enhanced and the particle's optical absorption has a maximum at the plasmon resonant frequency. Surface plasmon resonance can also be tuned based on the shape of the nanoparticle.[1] The plasmon frequency can be related to the metal dielectric constant.[1] The enhancement falls off quickly with distance from the surface and, for noble metal nanoparticles, the resonance occurs at visible wavelengths.[2] Localized surface plasmon resonance creates brilliant colors in metal colloidal solutions.[3]
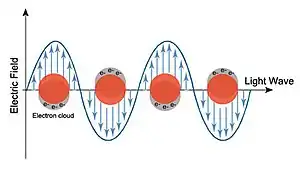
For metals like silver and gold, the oscillation frequency is also affected by the electrons in d-orbitals. Silver is a popular choice in plasmonics, which studies the effect of coupling light to charges, because it can support a surface plasmon over a wide range of wavelengths (300-1200 nm), and its peak absorption wavelength is easily changed.[2] For instance, the peak absorption wavelength of triangular silver nanoparticles was altered by changing the corner sharpness of the triangles. It underwent a blue-shift as corner sharpness of the triangles decreased.[4] Additionally, peak absorption wavelength underwent a red-shift as a larger amount of reducing agent (HAuCl4) was added and porosity of the particles increased.[3] For semiconductor nanoparticles, the maximum optical absorption is often in the near-infrared and mid-infrared region.[5][6]
Propagating surface plasmons
Localized surface plasmons are distinct from propagating surface plasmons. In localized surface plasmons, the electron cloud oscillates collectively. In propagating surface plasmons, the surface plasmon propagates back and forth between the ends of the structure. Propagating surface plasmons also need to have at least one dimension that is close to or longer than the wavelength of incident light. The waves created in propagating surface plasmons can also be tuned by controlling the geometry of the metal nanostructure.[2]
Characterization and study of localized surface plasmons
A goal of plasmonics is to understand and manipulate surface plasmons at the nano-scale, so characterization of surface plasmons is important. Some techniques frequently used to characterize surface plasmons are dark-field microscopy, UV-vis-NIR spectroscopy, and surface-enhanced Raman scattering (SERS).[2] With dark-field microscopy, it is possible to monitor the spectrum of an individual metal nanostructure as the incident light polarization, wavelength, or variations in the dielectric environment is changed.[7]
Applications
.jpg.webp)
The plasmon resonant frequency is highly sensitive to the refractive index of the environment; a change in refractive index results in a shift in the resonant frequency. As the resonant frequency is easy to measure, this allows LSP nanoparticles to be used for nanoscale sensing applications.[8] Also, nanoparticles exhibiting strong LSP properties, such as gold nanorods, could enhance the signal in surface plasmon resonance sensing.[9][10] Nanostructures exhibiting LSP resonances are used to enhance signals in modern analytical techniques based on spectroscopy. Other applications that rely on efficient light to heat generation in the nanoscale are heat-assisted magnetic recording (HAMR), photothermal cancer therapy, and thermophotovoltaics.[11] So far, high efficiency applications using plasmonics have not been realized due to the high ohmic losses inside metals especially in the optical spectral range (visible and NIR).,[12][13] Additionally surface plasmons have been used to create super lenses, invisibility cloaks, and to improve quantum computing.[14][15][16] Another interesting area of research in plasmonics is the ability to turn plasmons "on" and "off" via modification of another molecule. The ability to turn plasmons on and off has important consequences for increasing sensitivity in detection methods.[2] Recently, a supramolecular chromophore was coupled with a metal nanostructure. This interaction changed the localized surface plasmon resonance properties of the silver nanostructure by increasing the absorption intensity.[17]
See also
References
- Kelly, K. Lance (December 21, 2002). "The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment". The Journal of Physical Chemistry B. 107 (3): 668–677. doi:10.1021/jp026731y.
- Rycenga, Matthew; Cobley, Claire M.; Zeng, Jie; Li, Weiyang; Moran, Christine H.; Zhang, Qiang; Qin, Dong; Xia, Younan (2011). "Controlling the Synthesis and Assembly of Silver Nanostructures for Plasmonic Applications". Chem. Rev. 111 (6): 3669–3712. doi:10.1021/cr100275d. PMC 3110991. PMID 21395318.
- Skrabalak, Sara E.; Au, Leslie; Li, Xingde; Xia, Younan (September 2007). "Facile synthesis of Ag nanocubes and Au nanocages". Nature Protocols. 2 (9): 2182–2190. doi:10.1038/nprot.2007.326. ISSN 1750-2799. PMID 17853874. S2CID 20587542.
- Zeng, Jie; Roberts, Stefan; Xia, Younan (2010). "Nanocrystal-Based Time–Temperature Indicators". Chemistry – A European Journal. 16 (42): 12559–12563. doi:10.1002/chem.201002665. ISSN 1521-3765. PMID 20945450.
- Liu, Xin; Swihart, Mark T. (2014). "Heavily-doped colloidal semiconductor and metal oxide nanocrystals: an emerging new class of plasmonic nanomaterials". Chem. Soc. Rev. 43 (11): 3908–3920. doi:10.1039/c3cs60417a. PMID 24566528.
- Zhou, Shu; Pi, Xiaodong; Ni, Zhenyi; Ding, Yi; Jiang, Yingying; Jin, Chuanhong; Delerue, Christophe; Yang, Deren; Nozaki, Tomohiro (2015). "Comparative study on the localized surface plasmon resonance of boron- and phosphorus-doped silicon nanocrystals". ACS Nano. 9 (1): 378–386. doi:10.1021/nn505416r. PMID 25551330.
- Haes, Amanda J.; Van Duyne, Richard P. (2004-08-01). "A unified view of propagating and localized surface plasmon resonance biosensors". Analytical and Bioanalytical Chemistry. 379 (7): 920–930. doi:10.1007/s00216-004-2708-9. ISSN 1618-2650. PMID 15338088. S2CID 4814291.
- Mayer, Kathryn M.; Hafner, Jason H. (2011). "Localized Surface Plasmon Resonance Sensors". Chemical Reviews. Plasmonics (111): 3828–3857. doi:10.1021/cr100313v. PMID 21648956.
- Loo, Jacky Fong-Chuen; Yang, Chengbin; Tsang, Hing Lun; Lau, Pui Man; Yong, Ken-Tye; Ho, Ho Pui; Kong, Siu Kai (2017). "An Aptamer Bio-barCode (ABC) assay using SPR, RNase H, and probes with RNA and gold-nanorods for anti-cancer drug screening". The Analyst. 142 (19): 3579–3587. Bibcode:2017Ana...142.3579L. doi:10.1039/C7AN01026E. ISSN 0003-2654. PMID 28852760.
- Law, Wing-Cheung; Yong, Ken-Tye; Baev, Alexander; Hu, Rui; Prasad, Paras N. (2009-10-12). "Nanoparticle enhanced surface plasmon resonance biosensing: Application of gold nanorods". Optics Express. 17 (21): 19041–19046. Bibcode:2009OExpr..1719041L. doi:10.1364/OE.17.019041. ISSN 1094-4087. PMID 20372639.
- ElKabbash, Mohamed; et al. (2017). "Tunable Black Gold: Controlling the Near-Field Coupling of Immobilized Au Nanoparticles Embedded in Mesoporous Silica Capsules". Advanced Optical Materials. 5 (21): 1700617. doi:10.1002/adom.201700617. S2CID 103781835.
- Khurgin, Jacob (2015). "How to deal with the loss in plasmonics and metamaterials". Nature Nanotechnology. 10 (1): 2–6. arXiv:1411.6577. Bibcode:2015NatNa..10....2K. doi:10.1038/nnano.2014.310. PMID 25559961. S2CID 6906889.
- ElKabbash, Mohamed; et al. (2017). "Ultrafast transient optical loss dynamics in exciton–plasmon nano-assemblies". Nanoscale. 9 (19): 6558–6566. doi:10.1039/c7nr01512g. hdl:11693/37238. PMID 28470299.
- Fang, Nicholas; Lee, Hyesog; Sun, Cheng; Zhang, Xiang (2005-04-22). "Sub-Diffraction-Limited Optical Imaging with a Silver Superlens". Science. 308 (5721): 534–537. Bibcode:2005Sci...308..534F. doi:10.1126/science.1108759. ISSN 0036-8075. PMID 15845849. S2CID 1085807.
- Shalaev, Vladimir M. (January 2007). "Optical negative-index metamaterials". Nature Photonics. 1 (1): 41–48. Bibcode:2007NaPho...1...41S. doi:10.1038/nphoton.2006.49. ISSN 1749-4893. S2CID 170678.
- Chang, D. E.; Sørensen, A. S.; Hemmer, P. R.; Lukin, M. D. (2006-08-03). "Quantum Optics with Surface Plasmons". Physical Review Letters. 97 (5): 053002. arXiv:quant-ph/0506117. Bibcode:2006PhRvL..97e3002C. doi:10.1103/PhysRevLett.97.053002. PMID 17026098. S2CID 7782449.
- Zhou, Haibo; Yang, Danting; Ivleva, Natalia P.; Mircescu, Nicoleta E.; Schubert, Sören; Niessner, Reinhard; Wieser, Andreas; Haisch, Christoph (2015-07-07). "Label-Free in Situ Discrimination of Live and Dead Bacteria by Surface-Enhanced Raman Scattering". Analytical Chemistry. 87 (13): 6553–6561. doi:10.1021/acs.analchem.5b01271. ISSN 0003-2700. PMID 26017069.