Osteomalacia
Osteomalacia is a disease characterized by the softening of the bones caused by impaired bone metabolism primarily due to inadequate levels of available phosphate, calcium, and vitamin D, or because of resorption of calcium. The impairment of bone metabolism causes inadequate bone mineralization. Osteomalacia in children is known as rickets, and because of this, use of the term "osteomalacia" is often restricted to the milder, adult form of the disease. Signs and symptoms can include diffuse body pains, muscle weakness, and fragility of the bones. In addition to low systemic levels of circulating mineral ions (for example, caused by vitamin D deficiency or renal phosphate wasting) that result in decreased bone and tooth mineralization, accumulation of mineralization-inhibiting proteins and peptides (such as osteopontin and ASARM peptides), and small inhibitory molecules (such as pyrophosphate), can occur in the extracellular matrix of bones and teeth, contributing locally to cause matrix hypomineralization (osteomalacia/odontomalacia).[1][2][3][4][5][6][7] A relationship describing local, physiologic double-negative (inhibiting inhibitors) regulation of mineralization has been termed the Stenciling Principle of mineralization, whereby enzyme-substrate pairs imprint mineralization patterns into the extracellular matrix (most notably described for bone) by degrading mineralization inhibitors (e.g. TNAP/TNSALP/ALPL enzyme degrading the pyrophosphate inhibition, and PHEX enzyme degrading the osteopontin inhibition).[8][9] The Stenciling Principle for mineralization is particularly relevant to the osteomalacia and odontomalacia observed in hypophosphatasia (HPP) and X-linked hypophosphatemia (XLH).
| Osteomalacia | |
|---|---|
 | |
| Cholecalciferol (Vitamin D3), deficiency of which is the most common cause of Osteomalacia | |
| Specialty | Rheumatology |
The most common cause of osteomalacia is a deficiency of vitamin D, which is normally derived from sunlight exposure and, to a lesser extent, from the diet.[10] The most specific screening test for vitamin D deficiency in otherwise healthy individuals is a serum 25(OH)D level.[11] Less common causes of osteomalacia can include hereditary deficiencies of vitamin D or phosphate (which would typically be identified in childhood) or malignancy.
Vitamin D and calcium supplements are measures that can be used to prevent and treat osteomalacia. Vitamin D should always be administered in conjunction with calcium supplementation (as the pair work together in the body) since most of the consequences of vitamin D deficiency are a result of impaired mineral ion homeostasis.[11]
Nursing home residents and the homebound elderly population are at particular risk for vitamin D deficiency, as these populations typically receive little sun exposure. In addition, both the efficiency of vitamin D synthesis in the skin and the absorption of vitamin D from the intestine decline with age, thus further increasing the risk in these populations. Other groups at risk include individuals with malabsorption secondary to gastrointestinal bypass surgery or celiac disease, and individuals who immigrate from warm climates to cold climates, especially women who wear traditional veils or dresses that prevent sun exposure.[12]
Signs and symptoms
Osteomalacia is a generalized bone condition in which there is inadequate mineralization of the bone. Many of the effects of the disease overlap with the more common osteoporosis, but the two diseases are significantly different. There are two main causes of osteomalacia:
- insufficient calcium absorption from the intestine because of lack of dietary calcium or a deficiency of, or resistance to, the action of vitamin D, or due to undiagnosed celiac disease.[13]
- phosphate deficiency caused by increased renal losses.
Symptoms:
- Diffuse joint and bone pain (especially of spine, pelvis, and legs)
- Muscle weakness
- Difficulty walking, often with a waddling gait
- Hypocalcemia (positive Chvostek sign)
- Compressed vertebrae and diminished stature
- Pelvic flattening
- Weak, soft bones
- Easy fracturing
- Bending of bones
Osteomalacia in adults starts insidiously as aches and pains in the lumbar (lower back) region and thighs before spreading to the arms and ribs. The pain is symmetrical, non-radiating and accompanied by sensitivity in the involved bones. Proximal muscles are weak, and there is difficulty in climbing upstairs and getting up from a squatting position.[14] As a result of demineralization, the bones become less rigid. Physical signs include deformities like triradiate pelvis[15] and lordosis. The patient has a typical "waddling" gait. However, these physical signs may derive from a previous osteomalacial state, since bones do not regain their original shape after they become deformed.
Pathologic fractures due to weight bearing may develop. Most of the time, the only alleged symptom is chronic fatigue, while bone aches are not spontaneous but only revealed by pressure or shocks.[16] It differs from renal osteodystrophy, where the latter shows hyperphosphatemia.
Causes
The causes of adult osteomalacia are varied, but ultimately result in a vitamin D deficiency:
- Insufficient nutritional quantities or faulty metabolism of vitamin D or phosphorus
- Renal tubular acidosis
- Malnutrition during pregnancy
- Malabsorption syndrome
- Hypophosphatemia[17]
- Chronic kidney failure
- Tumor-induced osteomalacia (Oncogenic osteomalacia)
- Long-term anticonvulsant therapy[18]
- Celiac disease[19]
- Cadmium poisoning, itai-itai disease
Diagnosis
Biochemical findings
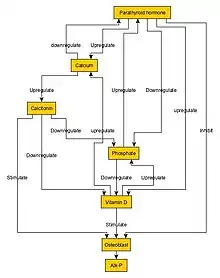
Biochemical features are similar to those of rickets. The major factor is an abnormally low vitamin D concentration in blood serum.[20] Major typical biochemical findings include:[21]
- Low serum and urinary calcium
- Low serum phosphate, except in cases of renal osteodystrophy
- Elevated serum alkaline phosphatase (due to an increase in compensatory osteoblast activity)
- Elevated parathyroid hormone (due to low calcium)
Furthermore, a technetium bone scan will show increased activity (also due to increased osteoblasts).
| Condition | Calcium | Phosphate | Alkaline phosphatase | Parathyroid hormone | Comments |
|---|---|---|---|---|---|
| Osteopenia | unaffected | unaffected | normal | unaffected | decreased bone mass |
| Osteopetrosis | unaffected | unaffected | elevated | unaffected | thick dense bones also known as marble bone |
| Osteomalacia and rickets | decreased | decreased | elevated | elevated | soft bones |
| Osteitis fibrosa cystica | elevated | decreased | elevated | elevated | brown tumors |
| Paget's disease of bone | unaffected | unaffected | variable (depending on stage of disease) | unaffected | abnormal bone architecture |
Radiographic characteristics
Radiological appearances include:
- Pseudofractures, also called Looser's zones.
- Protrusio acetabuli, a hip joint disorder
Prevention
Prevention of osteomalacia rests on having an adequate intake of vitamin D and calcium, or other treatments if the osteomalacia hereditary (genetic). Vitamin D3 Supplementation is often needed due to the scarcity of Vitamin D sources in the modern diet.[22]
Treatment
Nutritional osteomalacia responds well to administration of 2,000-10,000 IU of vitamin D3 by mouth daily. Vitamin D3 (cholecalciferol) is typically absorbed more readily than vitamin D2 (ergocalciferol). Osteomalacia due to malabsorption may require treatment by injection or daily oral dosing[23] of significant amounts of vitamin D3.
Etymology
Osteomalacia is derived from Greek: osteo- which means "bone", and malacia which means "softness". In the past, the disease was also known as malacosteon and its Latin-derived equivalent, mollities ossium. Osteomalacia is associated with increase in osteoid maturation time.
See also
References
- McKee, MD; Buss, DJ; Reznikov, N (13 December 2021). "Mineral tessellation in bone and the stenciling principle for extracellular matrix mineralization". Journal of Structural Biology. 214 (1): 107823. doi:10.1016/j.jsb.2021.107823. PMID 34915130. S2CID 245187449.
- Buss, DJ; Reznikov, N; McKee, MD (1 November 2020). "Crossfibrillar mineral tessellation in normal and Hyp mouse bone as revealed by 3D FIB-SEM microscopy". Journal of Structural Biology. 212 (2): 107603. doi:10.1016/j.jsb.2020.107603. PMID 32805412. S2CID 221164596.
- Salmon, B; Bardet, C; Coyac, BR; Baroukh, B; Naji, J; Rowe, PS; Opsahl Vital, S; Linglart, A; Mckee, MD; Chaussain, C (August 2014). "Abnormal osteopontin and matrix extracellular phosphoglycoprotein localization, and odontoblast differentiation, in X-linked hypophosphatemic teeth". Connective Tissue Research. 55 Suppl 1: 79–82. doi:10.3109/03008207.2014.923864. PMID 25158186. S2CID 19702315.
- Boukpessi, T; Hoac, B; Coyac, BR; Leger, T; Garcia, C; Wicart, P; Whyte, MP; Glorieux, FH; Linglart, A; Chaussain, C; McKee, MD (21 November 2016). "Osteopontin and the dento-osseous pathobiology of X-linked hypophosphatemia". Bone. 95: 151–161. doi:10.1016/j.bone.2016.11.019. PMID 27884786.
- Barros, NM; Hoac, B; Neves, RL; Addison, WN; Assis, DM; Murshed, M; Carmona, AK; McKee, MD (March 2013). "Proteolytic processing of osteopontin by PHEX and accumulation of osteopontin fragments in Hyp mouse bone, the murine model of X-linked hypophosphatemia". Journal of Bone and Mineral Research. 28 (3): 688–99. doi:10.1002/jbmr.1766. PMID 22991293.
- McKee, MD; Hoac, B; Addison, WN; Barros, NM; Millán, JL; Chaussain, C (October 2013). "Extracellular matrix mineralization in periodontal tissues: Noncollagenous matrix proteins, enzymes, and relationship to hypophosphatasia and X-linked hypophosphatemia". Periodontology 2000. 63 (1): 102–22. doi:10.1111/prd.12029. PMC 3766584. PMID 23931057.
- Boukpessi, T; Gaucher, C; Léger, T; Salmon, B; Le Faouder, J; Willig, C; Rowe, PS; Garabédian, M; Meilhac, O; Chaussain, C (August 2010). "Abnormal presence of the matrix extracellular phosphoglycoprotein-derived acidic serine- and aspartate-rich motif peptide in human hypophosphatemic dentin". The American Journal of Pathology. 177 (2): 803–12. doi:10.2353/ajpath.2010.091231. PMC 2913338. PMID 20581062.
- Reznikov, N.; Hoac, B.; Buss, D. J.; Addison, W. N.; Barros NMT; McKee, M. D. (2020). "Biological stenciling of mineralization in the skeleton: Local enzymatic removal of inhibitors in the extracellular matrix". Bone. 138: 115447. doi:10.1016/j.bone.2020.115447. PMID 32454257. S2CID 218909350.
- McKee, M. D.; Buss, D. J.; Reznikov, N. (2022). "Mineral tessellation in bone and the Stenciling Principle for extracellular matrix mineralization". Journal of Structural Biology. 214 (1): 107823. doi:10.1016/j.jsb.2021.107823. PMID 34915130. S2CID 245187449.
- "Osteomalacia: MedlinePlus Medical Encyclopedia". medlineplus.gov.
- Longo, Dan L.; et al. (2012). Harrison's principles of internal medicine (18th ed.). New York: McGraw-Hill. ISBN 978-0-07174889-6.
- Kennel, KA; Drake, MT; Hurley, DL (August 2010). "Vitamin D deficiency in adults: when to test and how to treat". Mayo Clinic Proceedings. 85 (8): 752–7, quiz 757-8. doi:10.4065/mcp.2010.0138. PMC 2912737. PMID 20675513.
- Basu, R.A.; Elmer, K.; Babu, A.; Kelly, C. A. (2000). "Coeliac disease can still present with osteomalacia!". Rheumatology. 39 (3): 335–336. doi:10.1093/rheumatology/39.3.335. PMID 10788547.
- "Osteomalacia and Rickets". The Lecturio Medical Concept Library. Retrieved 23 August 2021.
- Chakravorty, N. K. (1980). "Triradiate deformity of the pelvis in Paget's disease of bone". Postgraduate Medical Journal. 56 (653): 213–5. doi:10.1136/pgmj.56.653.213. PMC 2425842. PMID 7393817.
- "Osteomalacia and Rickets". The Lecturio Medical Concept Library. Retrieved 23 August 2021.
- "Autoimmunity research foundation, Science behind Vitamin D". Retrieved 2011-07-19.
- Pack, Alison (2008). "Bone health in people with epilepsy: is it impaired and what are the risk factors". Seizure. 17 (2): 181–6. doi:10.1016/j.seizure.2007.11.020. PMID 18187347. S2CID 16490292.
- "Definition & Facts for Celiac Disease. What are the complications of celiac disease?". NIDDK. June 2016. Retrieved 26 May 2018.
- "Osteomalacia and Rickets". The Lecturio Medical Concept Library. Retrieved 23 August 2021.
- Holick, Michael F. (19 July 2007). "Vitamin D Deficiency". New England Journal of Medicine. 357 (3): 266–281. doi:10.1056/NEJMra070553. PMID 17634462.
- "Osteomalacia and Rickets". The Lecturio Medical Concept Library. Retrieved 23 August 2021.
- Eisman, John A. (1988). "6 Osteomalacia". Baillière's Clinical Endocrinology and Metabolism. 2 (1): 125–55. doi:10.1016/S0950-351X(88)80011-9. PMID 3044328.