Lucigenin
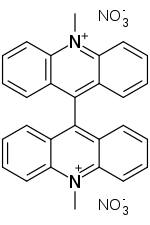
Lucigenin is an aromatic compound used in areas which include chemiluminescence. Its chemical name is bis-N-methylacridinium nitrate. It exhibits a bluish-green fluorescence.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
10,10′-Dimethyl[9,9′-biacridine]-10,10′-diium dinitrate | |
| Other names
Bis-N-methylacridinium nitrate; N,N′-Dimethyl-9,9'-bisacridinium nitrate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.017.295 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C28H22N4O6 | |
| Molar mass | 510.506 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
It is used as a probe for superoxide anion in biology, for its chemiluminescent properties.
Synthesis
It may be prepared from acridone.
There's also a route from toluene:

Synthesis of Lucigenin from Toluene. References in the image description.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.