Lumazine synthase
Lumazine synthase (EC 2.5.1.78, 6,7-dimethyl-8-ribityllumazine synthase, 6,7-dimethyl-8-ribityllumazine synthase 2, 6,7-dimethyl-8-ribityllumazine synthase 1, lumazine synthase 2, lumazine synthase 1, type I lumazine synthase, type II lumazine synthase, RIB4, MJ0303, RibH, Pbls, MbtLS, RibH1 protein, RibH2 protein, RibH1, RibH2) is an enzyme with systematic name 5-amino-6-(D-ribitylamino)uracil butanedionetransferase.[1] This enzyme catalyses the following chemical reaction
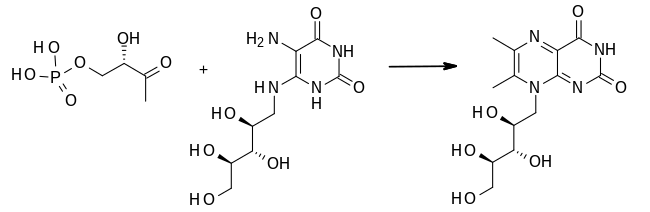
- 1-deoxy-L-glycero-tetrulose 4-phosphate + 5-amino-6-(D-ribitylamino)uracil 6,7-dimethyl-8-(D-ribityl)lumazine + 2 H2O + phosphate
| 6,7-dimethyl-8-ribityllumazine synthase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 2.5.1.78 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
This reaction is part of the biosynthesis of riboflavin (vitamin B2). Lumazine synthase is thus found in those organisms (plants, fungi and most microorganisms) which produce riboflavin.[2]
Depending on the species, 5, 10 or 60 copies of the enzyme bind together to form homomers. In the case of 60 copies, the enzyme units form a icosahedral hollow cage. In some bacteria, this cage contains another enzyme involved in the riboflavin synthesis, riboflavin synthase.[2]
These icosahedral cages have been investigated for use in drug delivery or as vaccines, delivering antigens.[2] Using directed evolution, Lumazine synthase has been modified so that it forms larger cages that preferentially package RNA molecules that code for the protein, akin to a virus capsid.[3]
References
- Kis K, Volk R, Bacher A (March 1995). "Biosynthesis of riboflavin. Studies on the reaction mechanism of 6,7-dimethyl-8-ribityllumazine synthase". Biochemistry. 34 (9): 2883–92. doi:10.1021/bi00009a019. PMID 7893702.
- Wei, Yangjie; Kumar, Prashant; Wahome, Newton; Mantis, Nicholas J.; Middaugh, C. Russell (2018). "Biomedical Applications of Lumazine Synthase" (PDF). Journal of Pharmaceutical Sciences. 107 (9): 2283–2296. doi:10.1016/j.xphs.2018.05.002. PMID 29763607.
- Tetter, Stephan; Terasaka, Naohiro; Steinauer, Angela; Bingham, Richard J.; Clark, Sam; Scott, Andrew J. P.; Patel, Nikesh; Leibundgut, Marc; Wroblewski, Emma; Ban, Nenad; Stockley, Peter G. (2021-06-11). "Evolution of a virus-like architecture and packaging mechanism in a repurposed bacterial protein". Science. 372 (6547): 1220–1224. doi:10.1126/science.abg2822. hdl:20.500.11850/490428. ISSN 0036-8075. PMID 34112695.
External links
- 6,7-dimethyl-8-ribityllumazine+synthase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)