Cardiothoracic surgery
Cardiothoracic surgery is the field of medicine involved in surgical treatment of organs inside the thoracic cavity — generally treatment of conditions of the heart (heart disease), lungs (lung disease), and other pleural or mediastinal structures.
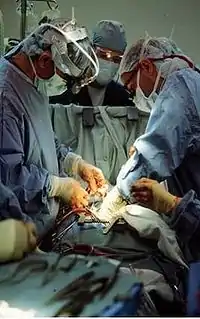 Cardiothoracic surgeon performs an operation. | |
| Occupation | |
|---|---|
| Names |
|
Occupation type | Specialty |
Activity sectors | Medicine, Surgery |
| Description | |
Education required |
|
Fields of employment | Hospitals, Clinics |
In most countries, cardiothoracic surgery is further subspecialized into cardiac surgery (involving the heart and the great vessels) and thoracic surgery (involving the lungs, esophagus, thymus, etc.); the exceptions are the United States, Australia, New Zealand, the United Kingdom, India and some European Union countries such as Portugal.[1]
Cardiothoracic surgery departments work especially closely with certain other specialties: cardiology departments and pulmonology/respirology departments (thoracic medicine, chest medicine).
Training
A cardiac surgery residency typically comprises anywhere from four to six years (or longer) of training to become a fully qualified surgeon.[2] Cardiac surgery training may be combined with thoracic surgery and/or vascular surgery and called cardiovascular (CV) / cardiothoracic (CT) / cardiovascular thoracic (CVT) surgery. Cardiac surgeons may enter a cardiac surgery residency directly from medical school, or first complete a general surgery residency followed by a fellowship. Cardiac surgeons may further sub-specialize cardiac surgery by doing a fellowship in a variety of topics including pediatric cardiac surgery, cardiac transplantation, adult-acquired heart disease, weak heart issues, and many more problems in the heart.
Australia and New Zealand
The highly competitive Surgical Education and Training (SET) program in Cardiothoracic Surgery is six years in duration, usually commencing several years after completing medical school. Training is administered and supervised via a bi-national (Australia and New Zealand) training program. Multiple examinations take place throughout the course of training, culminating in a final fellowship exam in the final year of training. Upon completion of training, surgeons are awarded a Fellowship of the Royal Australasian College of Surgeons (FRACS), denoting that they are qualified specialists. Trainees having completed a training program in General Surgery and have obtained their FRACS will have the option to complete fellowship training in Cardiothoracic Surgery of four years in duration, subject to college approval. It takes around eight to ten years minimum of post-graduate (post-medical school) training to qualify as a cardiothoracic surgeon. Competition for training places and for public (teaching) hospital places is very high currently, leading to concerns regarding workforce planning in Australia.
Canada
Historically, cardiac surgeons in Canada completed general surgery followed by a fellowship in CV / CT / CVT. During the 1990s, the Canadian cardiac surgery training programs changed to six-year "direct-entry" programs following medical school. The direct-entry format provides residents with experience related to cardiac surgery they would not receive in a general surgery program (e.g. echocardiography, coronary care unit, cardiac catheterization etc.). Residents in this program will also spend time training in thoracic and vascular surgery. Typically, this is followed by a fellowship in either Adult Cardiac Surgery, Heart Failure/Transplant, Minimally Invasive Cardiac Surgery, Aortic Surgery, Thoracic Surgery, Pediatric Cardiac Surgery or Cardiac ICU. Contemporary Canadian candidates completing general surgery and wishing to pursue cardiac surgery often complete a cardiothoracic surgery fellowship in the United States. The Royal College of Physicians and Surgeons of Canada also provides a three-year cardiac surgery fellowship for qualified general surgeons that is offered at several training sites including the University of Alberta, the University of British Columbia and the University of Toronto.
Thoracic surgery is its own separate 2–3 year fellowship of general or cardiac surgery in Canada.
Cardiac surgery programs in Canada:
- University of Alberta – 1 position
- University of British Columbia – 1 position
- University of Calgary – 1 position
- Dalhousie University – 1 position every other year
- Université Laval – 1 position every three years
- University of Manitoba – 1 position
- McGill University – 1 position every three years
- McMaster University – 1 position every other year
- Université de Montréal – 1 position every three years
- University of Ottawa – 1 position
- University of Toronto – 1 position
- Western University – 1 position
United Kingdom
In the United Kingdom, cardiac surgeons are trained by direct specialty training. Trainees may choose to subspecialise in areas such as aortic surgery, adult cardiac surgery, thoracic surgery, pediatric cardiothoracic surgery, and adult congenital surgery.
United States
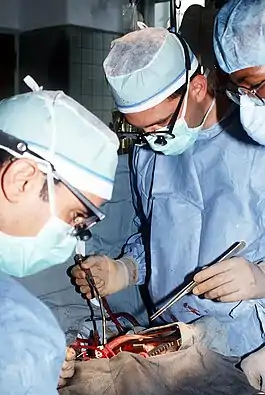
Cardiac surgery training in the United States is combined with general thoracic surgery and called cardiothoracic surgery or thoracic surgery. A cardiothoracic surgeon in the U.S. is a physician who first completes a general surgery residency (typically 5–7 years), followed by a cardiothoracic surgery fellowship (typically 2–3 years). The cardiothoracic surgery fellowship typically spans two or three years, but certification is based on the number of surgeries performed as the operating surgeon, not the time spent in the program, in addition to passing rigorous board certification tests. Two other pathways to shorten the duration of training have been developed: (1) a combined general-thoracic surgery residency consisting of four years of general surgery training and three years of cardiothoracic training at the same institution and (2) an integrated six-year cardiothoracic residency (in place of the general surgery residency plus cardiothoracic residency), which have each been established at many programs (over 20).[3] Applicants match into the integrated six-year (I-6) programs directly out of medical school, and the application process has been extremely competitive for these positions as there were approximately 160 applicants for 10 spots in the U.S. in 2010. As of May 2013, there are 20 approved programs, which include the following:
Integrated six-year Cardiothoracic Surgery programs in the United States:
- Medical College of Wisconsin
- Stanford University – two positions
- University of North Carolina at Chapel Hill
- University of Virginia
- Columbia University – two positions
- University of Pennsylvania
- University of Pittsburgh – two positions
- University of Washington
- Northwestern University
- Mount Sinai Hospital, New York
- University of Maryland
- University of California, Los Angeles UCLA – two resident positions, one Transplant Fellowship; one Congenital resident position
- University of Texas Health Science Center at San Antonio
- Medical University of South Carolina
- University of Southern California – two positions
- University of Rochester
- University of California, Davis
- Indiana University
- University of Kentucky
- Emory University
- University of Michigan
- Yale University
The American Board of Thoracic Surgery offers a special pathway certificate in congenital cardiac surgery which typically requires an additional year of fellowship. This formal certificate is unique because congenital cardiac surgeons in other countries do not have formal evaluation and recognition of pediatric training by a licensing body.
Cardiac surgery
| Cardiac surgery | |
|---|---|
 Two cardiac surgeons performing a cardiac surgery known as coronary artery bypass surgery. Note the use of a steel retractor to forcefully maintain the exposure of the patient's heart. | |
| ICD-9-CM | 35–37 |
| MeSH | D006348 |
| OPS-301 code | 5-35...5-37 |
The earliest operations on the pericardium (the sac that surrounds the heart) took place in the 19th century and were performed by Francisco Romero (1801)[4] Dominique Jean Larrey, Henry Dalton, and Daniel Hale Williams.[5] The first surgery on the heart itself was performed by Norwegian surgeon Axel Cappelen on 4 September 1895 at Rikshospitalet in Kristiania, now Oslo. He ligated a bleeding coronary artery in a 24-year-old man who had been stabbed in the left axilla and was in deep shock upon arrival. Access was through a left thoracotomy. The patient awoke and seemed fine for 24 hours, but became ill with increasing temperature and he ultimately died from what the post mortem proved to be mediastinitis on the third postoperative day.[6][7] The first successful surgery of the heart, performed without any complications, was by Ludwig Rehn of Frankfurt, Germany, who repaired a stab wound to the right ventricle on September 7, 1896.[8][9]
Surgery in great vessels (aortic coarctation repair, Blalock-Taussig shunt creation, closure of patent ductus arteriosus) became common after the turn of the century and falls in the domain of cardiac surgery, but technically cannot be considered heart surgery. One of the more commonly known cardiac surgery procedures is the coronary artery bypass graft (CABG), also known as "bypass surgery." In this procedure, vessels from elsewhere in the patient's body are harvested, and grafted to the coronary arteries to bypass blockages and improve the blood supply to the heart muscle.
Early approaches to heart malformations
In 1925 operations on the heart valves were unknown. Henry Souttar operated successfully on a young woman with mitral stenosis. He made an opening in the appendage of the left atrium and inserted a finger into this chamber in order to palpate and explore the damaged mitral valve. The patient survived for several years[10] but Souttar's physician colleagues at that time decided the procedure was not justified and he could not continue.[11][12]
Cardiac surgery changed significantly after World War II. In 1948 four surgeons carried out successful operations for mitral stenosis resulting from rheumatic fever. Horace Smithy (1914–1948) of Charlotte, revived an operation due to Dr Dwight Harken of the Peter Bent Brigham Hospital using a punch to remove a portion of the mitral valve. Charles Bailey (1910–1993) at the Hahnemann Hospital, Philadelphia, Dwight Harken in Boston and Russell Brock at Guy's Hospital all adopted Souttar's method. All these men started work independently of each other, within a few months. This time Souttar's technique was widely adopted although there were modifications.[11][12]
In 1947 Thomas Holmes Sellors (1902–1987) of the Middlesex Hospital operated on a Fallot's Tetralogy patient with pulmonary stenosis and successfully divided the stenosed pulmonary valve. In 1948, Russell Brock, probably unaware of Sellor's work, used a specially designed dilator in three cases of pulmonary stenosis. Later in 1948 he designed a punch to resect the infundibular muscle stenosis which is often associated with Fallot's Tetralogy. Many thousands of these "blind" operations were performed until the introduction of heart bypass made direct surgery on valves possible.[11]
Open heart surgery
Open heart surgery is a procedure in which the patient's heart is opened and surgery is performed on the internal structures of the heart. It was discovered by Wilfred G. Bigelow of the University of Toronto that the repair of intracardiac pathologies was better done with a bloodless and motionless environment, which means that the heart should be stopped and drained of blood. The first successful intracardiac correction of a congenital heart defect using hypothermia was performed by C. Walton Lillehei and F. John Lewis at the University of Minnesota on September 2, 1952. The following year, Soviet surgeon Aleksandr Aleksandrovich Vishnevskiy conducted the first cardiac surgery under local anesthesia.
Surgeons realized the limitations of hypothermia – complex intracardiac repairs take more time and the patient needs blood flow to the body, particularly to the brain. The patient needs the function of the heart and lungs provided by an artificial method, hence the term cardiopulmonary bypass. John Heysham Gibbon at Jefferson Medical School in Philadelphia reported in 1953 the first successful use of extracorporeal circulation by means of an oxygenator, but he abandoned the method, disappointed by subsequent failures. In 1954 Lillehei realized a successful series of operations with the controlled cross-circulation technique in which the patient's mother or father was used as a 'heart-lung machine'. John W. Kirklin at the Mayo Clinic in Rochester, Minnesota started using a Gibbon type pump-oxygenator in a series of successful operations, and was soon followed by surgeons in various parts of the world.
Nazih Zuhdi performed the first total intentional hemodilution open heart surgery on Terry Gene Nix, age 7, on February 25, 1960, at Mercy Hospital, Oklahoma City, OK. The operation was a success; however, Nix died three years later in 1963.[13] In March, 1961, Zuhdi, Carey, and Greer, performed open heart surgery on a child, age 3+1⁄2, using the total intentional hemodilution machine. In 1985 Zuhdi performed Oklahoma's first successful heart transplant on Nancy Rogers at Baptist Hospital. The transplant was successful, but Rogers, who had cancer, died from an infection 54 days after surgery.[14]
Modern beating-heart surgery
Since the 1990s, surgeons have begun to perform "off-pump bypass surgery" – coronary artery bypass surgery without the aforementioned cardiopulmonary bypass. In these operations, the heart is beating during surgery, but is stabilized to provide an almost still work area in which to connect the conduit vessel that bypasses the blockage; in the U.S., most conduit vessels are harvested endoscopically, using a technique known as endoscopic vessel harvesting (EVH).
Some researchers believe that the off-pump approach results in fewer post-operative complications, such as postperfusion syndrome, and better overall results. Study results are controversial as of 2007, the surgeon's preference and hospital results still play a major role.
Minimally invasive surgery
A new form of heart surgery that has grown in popularity is robot-assisted heart surgery. This is where a machine is used to perform surgery while being controlled by the heart surgeon. The main advantage to this is the size of the incision made in the patient. Instead of an incision being at least big enough for the surgeon to put his hands inside, it does not have to be bigger than 3 small holes for the robot's much smaller "hands" to get through.
Pediatric cardiovascular surgery
Pediatric cardiovascular surgery is surgery of the heart of children. The first operations to repair cardio-vascular[15] defects in children were performed by Clarence Crafoord in Sweden when he repaired coarctation of the aorta in a 12-year-old boy.[16] The first attempts to palliate congenital heart disease were performed by Alfred Blalock with the assistance of William Longmire, Denton Cooley, and Blalock's experienced technician, Vivien Thomas in 1944 at Johns Hopkins Hospital.[17] Techniques for repair of congenital heart defects without the use of a bypass machine were developed in the late 1940s and early 1950s. Among them was an open repair of an atrial septal defect using hypothermia, inflow occlusion and direct vision in a 5-year-old child performed in 1952 by Lewis and Tauffe. C. Walter Lillihei used cross-circulation between a boy and his father to maintain perfusion while performing a direct repair of a ventricular septal defect in a 4-year-old child in 1954.[18] He continued to use cross-circulation and performed the first corrections of tetralogy of Fallot and presented those results in 1955 at the American Surgical Association. In the long-run, pediatric cardiovascular surgery would rely on the cardiopulmonary bypass machine developed by Gibbon and Lillehei as noted above.
Risks of cardiac surgery
The development of cardiac surgery and cardiopulmonary bypass techniques has reduced the mortality rates of these surgeries to relatively low ranks. For instance, repairs of congenital heart defects are currently estimated to have 4–6% mortality rates.[19][20] A major concern with cardiac surgery is the incidence of neurological damage. Stroke occurs in 5% of all people undergoing cardiac surgery, and is higher in patients at risk for stroke.[21] A more subtle constellation of neurocognitive deficits attributed to cardiopulmonary bypass is known as postperfusion syndrome, sometimes called "pumphead". The symptoms of postperfusion syndrome were initially felt to be permanent,[22] but were shown to be transient with no permanent neurological impairment.[23]
In order to assess the performance of surgical units and individual surgeons, a popular risk model has been created called the EuroSCORE. This takes a number of health factors from a patient and using precalculated logistic regression coefficients attempts to give a percentage chance of survival to discharge. Within the UK this EuroSCORE was used to give a breakdown of all the centres for cardiothoracic surgery and to give some indication of whether the units and their individuals surgeons performed within an acceptable range. The results are available on the CQC website.[24] The precise methodology used has however not been published to date nor has the raw data on which the results are based.
Infection represents the primary non-cardiac complication from cardiothoracic surgery. Infections can include mediastinitis, infectious myo- or pericarditis, endocarditis, cardiac device infection, pneumonia, empyema, and bloodstream infections. Clostridium difficile colitis can also develop when prophylactic or post-operative antibiotics are used.
Post operative patients of cardiothoracic surgery are at risk of nausea, vomiting, dysphagia and aspiration pneumonia.[25]
Thoracic surgery
A pleurectomy is a surgical procedures in which part of the pleura is removed. It is sometimes used in the treatment of pneumothorax and mesothelioma.[26] In case of pneumothorax, only the apical and the diaphragmatic portions of the parietal pleura are removed.
Lung volume reduction surgery
Lung volume reduction surgery, or LVRS, can improve the quality of life for certain patients with COPD of emphysematous type, when other treatment options are not enough. Parts of the lung that are particularly damaged by emphysema are removed, allowing the remaining, relatively good lung to expand and work more efficiently. The beneficial effects are correlated with the achieved reduction in residual volume.[27] Conventional LVRS involves resection of the most severely affected areas of emphysematous, non-bullous lung (aim is for 20–30%). This is a surgical option involving a mini-thoracotomy for patients in end stage COPD due to underlying emphysema, and can improve lung elastic recoil as well as diaphragmatic function.
The National Emphysema Treatment Trial (NETT) was a large multicentre study (N = 1218) comparing LVRS with non-surgical treatment. Results suggested that there was no overall survival advantage in the LVRS group, except for mainly upper-lobe emphysema + poor exercise capacity, and significant improvements were seen in exercise capacity in the LVRS group.[28] Later studies have shown a wider scope of treatment with better outcomes.[29]
Possible complications of LVRS include prolonged air leak (mean duration post surgery until all chest tubes removed is 10.9 ± 8.0 days.[30]
In people who have a predominantly upper lobe emphysema, lung volume reduction surgery could result in better health status and lung function, though it also increases the risk of early mortality and adverse events.[31]
LVRS is used widely in Europe, though its application in the United States is mostly experimental.[32]
A less invasive treatment is available as a bronchoscopic lung volume reduction procedure.[33]
Lung cancer surgery
Not all lung cancers are suitable for surgery. The stage, location and cell type are important limiting factors. In addition, people who are very ill with a poor performance status or who have inadequate pulmonary reserve would be unlikely to survive. Even with careful selection, the overall operative death rate is about 4.4%.[34]
In non-small cell lung cancer staging, stages IA, IB, IIA, and IIB are suitable for surgical resection.[35]
Pulmonary reserve is measured by spirometry. If there is no evidence of undue shortness of breath or diffuse parenchymal lung disease, and the FEV1 exceeds 2 litres or 80% of predicted, the person is fit for pneumonectomy. If the FEV1 exceeds 1.5 litres, the patient is fit for lobectomy.[36]
There is weak evidence to indicate that participation in exercise programs before lung cancer surgery may reduce the risk of complications after surgery.[37]
Complications
A prolonged air leak (PAL) can occur in 8–25% of people following lung cancer surgery.[38][39] This complication delays chest tube removal and is associated with an increased length of hospital stay following a lung resection (lung cancer surgery).[40][41] The use of surgical sealants may reduce the incidence of prolonged air leaks, however, this intervention alone has not been shown to results in a decreased length of hospital stay following lung cancer surgery.[42]
There is no strong evidence to support using non-invasive positive pressure ventilation following lung cancer surgery to reduce pulmonary complications.[43]
Types
- Lobectomy (removal of a lobe of the lung)[44]
- Sublobar resection (removal of part of lobe of the lung)
- Segmentectomy (removal of an anatomic division of a particular lobe of the lung)
- Pneumonectomy (removal of an entire lung)
- Wedge resection
- Sleeve/bronchoplastic resection (removal of an associated tubular section of the associated main bronchial passage during lobectomy with subsequent reconstruction of the bronchial passage)
- VATS lobectomy (minimally invasive approach to lobectomy that may allow for diminished pain, quicker return to full activity, and diminished hospital costs)[45][46]
- esophagectomy (removal of the esophagus)
See also
References
- "Portuguese Ordem dos Médicos – Medical specialties" (in Portuguese). Archived from the original on 23 January 2012.
- "Thoracic Surgery Specialty Description". American Medical Association. Retrieved 28 September 2020.
- "Integrated Thoracic Surgery Residency Programs – TSDA". www.tsda.org. Archived from the original on 31 January 2018. Retrieved 8 May 2018.
- Aris A (1997). "Francisco Romero, the first heart surgeon". Ann Thorac Surg. 64 (3): 870–1. doi:10.1016/s0003-4975(97)00760-1. PMID 9307502.
- "Pioneers in Academic Surgery – Opening Doors: Contemporary African American Academic Surgeons". Archived from the original on 29 March 2016. Retrieved 12 February 2016. Pioneers in Academic Surgery, U.S. National Library of Medicine
- Landmarks in Cardiac Surgery by Stephen Westaby, Cecil Bosher, ISBN 1-899066-54-3
- Raouf, N. (2019). "Tidsskrift for den Norske Legeforening". Tidsskrift for den Norske Laegeforening. 139 (14). doi:10.4045/tidsskr.19.0505. PMID 31592616.
- Absolon KB, Naficy MA (2002). First successful cardiac operation in a human, 1896: a documentation: the life, the times, and the work of Ludwig Rehn (1849–1930). Rockville, MD : Kabel, 2002
- Johnson SL (1970). History of Cardiac Surgery, 1896–1955. Baltimore: Johns Hopkins Press. p. 5.
- Dictionary of National Biography – Henry Souttar (2004–08)
- Harold Ellis (2000) A History of Surgery, page 223+
- Lawrence H Cohn (2007), Cardiac Surgery in the Adult, page 6+
- Warren, Cliff, Dr. Nazih Zuhdi – His Scientific Work Made All Paths Lead to Oklahoma City, in Distinctly Oklahoma, November, 2007, p. 30–33
- "NDepth: Dr.Nazih Zuhdi, the Legendary Heart Surgeon | Newsok.com". Archived from the original on 25 April 2012. Retrieved 16 April 2012. Dr. Nazih Zuhdi, the Legendary Heart Surgeon, The Oklahoman, Jan 2010
- Wikipedia: Coarctation of the Aorta. Coarctation is not cardiac (i.e., heart) but is a narrowing of the aorta, a great vessel near the heart
- Crafoord C, Nyhlin G (1945). "Congenital coarctation of the aorta and its surgical management". J Thorac Surg. 14: 347–361. doi:10.1016/S0096-5588(20)31801-8.
- Blalock A, Taussig HB (1948). "The surgical treatment of malformations of the heart in which there is pulmonary stenosis or pulmonary atresia". JAMA. 128: 189–202. doi:10.1001/jama.1945.02860200029009.
- Lillehei CW, Cohen M, Warden HE; et al. (1955). "The results of direct vision closure of ventricular septal defects in eight patients by means of controlled cross circulation". Surgery, Gynecology, and Obstetrics. October (4): 447–66. PMID 13256320.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Stark J, Gallivan S, Lovegrove J, Hamilton JR, Monro JL, Pollock JC, Watterson KG (2000). "Mortality rates after surgery for congenital heart defects in children and surgeons' performance". Lancet. 355 (9208): 1004–7. doi:10.1016/s0140-6736(00)90001-1. PMID 10768449. S2CID 26116465.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Klitzner, Thomas S.; Lee, Maggie; Rodriguez, Sandra; Chang, Ruey-Kang R. (2006). "Sex-related Disparity in Surgical Mortality among Pediatric Patients". Congenital Heart Disease. 1 (3): 77–88. doi:10.1111/j.1747-0803.2006.00013.x. PMID 18377550.
- Jan Bucerius; Jan F. Gummert; Michael A. Borger; Thomas Walther; et al. (2003). "Stroke after cardiac surgery: a risk factor analysis of 16,184 consecutive adult patients". The Annals of Thoracic Surgery. 75 (2): 472–478. doi:10.1016/S0003-4975(02)04370-9. PMID 12607656.
- Newman M; Kirchner J; Phillips-Bute B; Gaver V; et al. (2001). "Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery". N Engl J Med. 344 (6): 395–402. doi:10.1056/NEJM200102083440601. PMID 11172175.
- Van Dijk D; Jansen E; Hijman R; Nierich A; et al. (2002). "Cognitive outcome after off-pump and on-pump coronary artery bypass graft surgery: a randomized trial". JAMA. 287 (11): 1405–12. doi:10.1001/jama.287.11.1405. PMID 11903027.
- "Heart Surgery in United Kingdom". Archived from the original on 5 November 2011. Retrieved 2011-10-21. CQC website for heart surgery outcomes in the UK for 3 years ending March 2009
- Ford C., McCormick D., Parkosewich J., et al. Safety and effectiveness of early oral hydration in patients after cardiothoracic surgery. Am. J. Crit. Care. 2020;29(4):292–300. doi:10.4037/ajcc2020841
- Aziz, Fahad (7 January 2017). "Pleurectomy". Medscape. Archived from the original on 6 October 2017. Retrieved 4 October 2017.
- Shah, Pallav L.; Weder, Walter; Kemp, Samuel V.; Herth, Felix J.; Slebos, Dirk-Jan; Geffen, Wouter H. van (7 February 2019). "Surgical and endoscopic interventions that reduce lung volume for emphysema: a systemic review and meta-analysis". The Lancet Respiratory Medicine. 7 (4): 313–324. doi:10.1016/S2213-2600(18)30431-4. ISSN 2213-2600. PMID 30744937. S2CID 73428098.
- Fishman, A; Martinez, F; Naunheim, K; Piantadosi, S; Wise, R; Ries, A; Weinmann, G; Wood, DE; National Emphysema Treatment Trial Research, Group (22 May 2003). "A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema". The New England Journal of Medicine. 348 (21): 2059–73. doi:10.1056/nejmoa030287. PMID 12759479.
- Caviezel, C; Schneiter, D; Opitz, I; Weder, W (August 2018). "Lung volume reduction surgery beyond the NETT selection criteria". Journal of Thoracic Disease. 10 (Suppl 23): S2748–S2753. doi:10.21037/jtd.2018.08.93. PMC 6129809. PMID 30210828.
- Hopkins, P. M.; Seale, H.; Walsh, J.; Tam, R.; Kermeen, F.; Bell, S.; McNeil, K. (1 February 2006). "51: Long term results post conventional lung volume reduction surgery exceeds outcome of lung transplantation for emphysema". The Journal of Heart and Lung Transplantation. 25 (2, Supplement): S61. doi:10.1016/j.healun.2005.11.053.
- van Agteren, JE; Carson, KV; Tiong, LU; Smith, BJ (14 October 2016). "Lung volume reduction surgery for diffuse emphysema". The Cochrane Database of Systematic Reviews. 2016 (10): CD001001. doi:10.1002/14651858.CD001001.pub3. PMC 6461146. PMID 27739074.
- Kronemyer, Bob (February 2018). "Four COPD Treatments to Watch". DrugTopics. 162 (2): 18.
- Gang Hou (30 December 2015). "Bronchoscopic lung volume reduction in chronic obstructive pulmonary disease:History and progress". Journal of Translational Internal Medicice. 3 (4): 147–150. doi:10.1515/jtim-2015-0023. PMC 4936455. PMID 27847904.
- Strand, TE; Rostad H; Damhuis RA; Norstein J (June 2007). "Risk factors for 30-day mortality after resection of lung cancer and prediction of their magnitude". Thorax. 62 (11): 991–7. doi:10.1136/thx.2007.079145. PMC 2117132. PMID 17573442.
- Mountain, CF (1997). "Revisions in the international system for staging lung cancer". Chest. 111 (6): 1710–1717. doi:10.1378/chest.111.6.1710. PMID 9187198. Archived from the original on 5 September 2003.
- Colice, GL; Shafazand S; Griffin JP; et al. (September 2007). "Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced-based clinical practice guidelines (2nd edition)". Chest. 132 (Suppl. 3): 161S–177S. doi:10.1378/chest.07-1359. PMID 17873167. Archived from the original on 14 April 2013.
- Cavalheri, Vinicius; Granger, Catherine (2017). "Preoperative exercise training for patients with non-small cell lung cancer". The Cochrane Database of Systematic Reviews. 2017 (6): CD012020. doi:10.1002/14651858.CD012020.pub2. ISSN 1469-493X. PMC 6481477. PMID 28589547.
- Novoa, Nuria M.; Jiménez, Marcelo F.; Varela, Gonzalo (2017). "When to Remove a Chest Tube". Thoracic Surgery Clinics. 27 (1): 41–46. doi:10.1016/j.thorsurg.2016.08.007. ISSN 1558-5069. PMID 27865326.
- Baringer, Kristina; Talbert, Steve (2017). "Chest drainage systems and management of air leaks after a pulmonary resection". Journal of Thoracic Disease. 9 (12): 5399–5403. doi:10.21037/jtd.2017.11.15. ISSN 2072-1439. PMC 5756963. PMID 29312751.
- Pompili, Cecilia; Miserocchi, Giuseppe (2016). "Air leak after lung resection: pathophysiology and patients' implications". Journal of Thoracic Disease. 8 (Suppl 1): S46–54. doi:10.3978/j.issn.2072-1439.2015.11.08. ISSN 2072-1439. PMC 4756241. PMID 26941970.
- Coughlin, Shaun M.; Emmerton-Coughlin, Heather M. A.; Malthaner, Richard (2012). "Management of chest tubes after pulmonary resection: a systematic review and meta-analysis". Canadian Journal of Surgery. 55 (4): 264–270. doi:10.1503/cjs.001411. ISSN 1488-2310. PMC 3404148. PMID 22854148.
- Belda-Sanchís, José; Serra-Mitjans, Mireia; Iglesias Sentis, Manuela; Rami, Ramon (20 January 2010). "Surgical sealant for preventing air leaks after pulmonary resections in patients with lung cancer". The Cochrane Database of Systematic Reviews. 2010 (1): CD003051. doi:10.1002/14651858.CD003051.pub3. ISSN 1469-493X. PMC 7138070. PMID 20091536.
- Torres, Maria Fs; Porfírio, Gustavo Jm; Carvalho, Alan Pv; Riera, Rachel (2019). "Non-invasive positive pressure ventilation for prevention of complications after pulmonary resection in lung cancer patients". The Cochrane Database of Systematic Reviews. 3 (3): CD010355. doi:10.1002/14651858.CD010355.pub3. ISSN 1469-493X. PMC 6402531. PMID 30840317.
- Fell, SC; TJ Kirby (2005). General Thoracic Surgery (sixth ed.). Lippincott Williams & Wilkins. pp. 433–457. ISBN 978-0-7817-3889-7.
- Nicastri DG, Wisnivesky JP, Litle VR, et al. (March 2008). "Thoracoscopic lobectomy: report on safety, discharge independence, pain, and chemotherapy tolerance". J Thorac Cardiovasc Surg. 135 (3): 642–7. doi:10.1016/j.jtcvs.2007.09.014. PMID 18329487.
- Casali G, Walker WS (March 2009). "Video-assisted thoracic surgery lobectomy: can we afford it?". Eur J Cardiothorac Surg. 35 (3): 423–8. doi:10.1016/j.ejcts.2008.11.008. PMID 19136272.