Lysidine (nucleoside)
Lysidine is an uncommon nucleoside, rarely seen outside of tRNA. It is a derivative of cytidine in which the carbonyl is replaced by the amino acid lysine. The third position in the anti-codon of the Isoleucine-specific tRNA, is typically changed from a cytidine which would pair with guanosine to a lysidine which will base pair with adenosine. Uridine could not be used at this position even though it is a conventional partner for adenosine since it will also "wobble base pair" with guanosine. So lysidine allows better translation fidelity.[1][2] Lysidine is denoted as L[3] or k2C[4] (lysine bound to C2 atom of cytidine).
 Lysidine base pairs with Adenosine in context of a Cytidine to Guanosine base pair. R = ribose. Arrows indicate hydrogen bonds going from hydrogens to bond acceptor. The notation for lysidine, L, is depicted above.
Lysidine base pairs with Adenosine in context of a Cytidine to Guanosine base pair. R = ribose. Arrows indicate hydrogen bonds going from hydrogens to bond acceptor. The notation for lysidine, L, is depicted above.
 | |
| Names | |
|---|---|
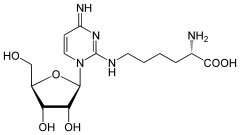
| IUPAC name
2-Amino-6-[4-amino-1-(3,4-dihydroxy-5-hydroxymethyloxolan-2-yl)-1H-pyrimidin-2-ylideneamino]hexanoic acid | |
| Other names
4-Amino-2-(N(6)-lysino)-1-ribofuranosylpyrimidine, 2-lysyl-cytidine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H25N5O6 | |
| Molar mass | 371.39 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- Nakanishi, Kotaro; Fukai, Shuya; Ikeuchi, Yoshiho; Soma, Akiko; Sekine, Yasuhiko; Suzuki, Tsutomu; Nureki, Osamu (24 May 2005). "Structural basis for lysidine formation by ATP pyrophosphatase accompanied by a lysine-specific loop and a tRNA-recognition domain". Proceedings of the National Academy of Sciences of the United States of America. 102 (21): 7487–7492. Bibcode:2005PNAS..102.7487N. doi:10.1073/pnas.0501003102. PMC 1140429. PMID 15894617.
- Salowe, Scott P.; Wiltsie, Judyann; Hawkins, Julio C.; Sonatore, Lisa M. (April 2009). "The Catalytic Flexibility of tRNAIle-lysidine Synthetase Can Generate Alternative tRNA Substrates for Isoleucyl-tRNA Synthetase". Journal of Biological Chemistry. 284 (15): 9656–9662. doi:10.1074/jbc.M809013200. PMC 2665086. PMID 19233850.
- Nakanishi, Kotaro; Bonnefond, Luc; Kimura, Satoshi; Suzuki, Tsutomu; Ishitani, Ryuichiro; Nureki, Osamu (October 2009). "Structural basis for translational fidelity ensured by transfer RNA lysidine synthetase". Nature. 461 (7267): 1144–1148. Bibcode:2009Natur.461.1144N. doi:10.1038/nature08474. PMID 19847269. S2CID 4426738.
- Sonawane, Kailas D.; Tewari, Ravindra (19 September 2008). "Conformational Preferences of Hypermodified Nucleoside Lysidine (k2C) Occurring at 'Wobble' Position in Anticodon Loop of tRNAIle". Nucleosides, Nucleotides & Nucleic Acids. 27 (10–11): 1158–1174. doi:10.1080/15257770802341475. PMID 18788046. S2CID 25220901.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.