HLA-DM
HLA-DM (human leukocyte antigen DM) is an intracellular protein involved in the mechanism of antigen presentation on antigen presenting cells (APCs) of the immune system.[2] It does this by assisting in peptide loading of major histocompatibility complex (MHC) class II membrane-bound proteins.[3] HLA-DM is encoded by the genes HLA-DMA and HLA-DMB.[4]
| major histocompatibility complex, class II, DM alpha | |||||||
|---|---|---|---|---|---|---|---|
 Crystallographic structure of human HLA-DM.[1] | |||||||
| Identifiers | |||||||
| Symbol | HLA-DMA | ||||||
| NCBI gene | 3108 | ||||||
| HGNC | 4934 | ||||||
| OMIM | 142855 | ||||||
| RefSeq | NM_006120 | ||||||
| UniProt | P28067 | ||||||
| Other data | |||||||
| Locus | Chr. 6 p21.3 | ||||||
| |||||||
| major histocompatibility complex, class II, DM beta | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | HLA-DMB | ||||||
| NCBI gene | 3109 | ||||||
| HGNC | 4935 | ||||||
| OMIM | 142856 | ||||||
| RefSeq | NM_002118 | ||||||
| UniProt | P28068 | ||||||
| Other data | |||||||
| Locus | Chr. 6 p21.3 | ||||||
| |||||||
HLA-DM is a molecular chaperone[5] that works in lysosomes and endosomes in cells of the immune system. It works in APCs like macrophages, dendritic cells, and B cells[6] by interacting with MHC class II molecules.[7] HLA-DM protects the MHC class II molecules from breaking down, and regulates which proteins or peptides bind to them as well.[5] This regulates how and when a peptide acts as an antigen initiating an immune response. Thus, HLA-DM is necessary for the immune system to respond effectively to a foreign invader. Impairment in HLA-DM function can result in immunodeficiency and autoimmune diseases.[8]
Genetics
The genes for HLA-DM are located in the MHCII region of the human chromosome 6.[2] The genes code for the alpha and beta chains that make up the protein.
The gene is nonpolymorphic.[8]
Function
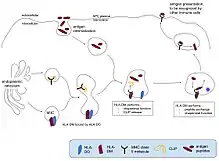
MHC class II + peptide interactions
HLA-DM is an integral protein in the mechanism regulating which antigens are presented extracellularly on APCs. It binds partially to the peptide-binding groove of MHC class II molecules.[9] This can affect how well your immune system responds to foreign invaders.[10]
HLA-DM is required to release CLIP from MHC class II molecules, to chaperone empty MHC molecules against denaturation, and to control proper loading and release of peptides at the peptide-binding groove.[11] It also interacts heavily with chaperone protein HLA-DO.[12] All of this ensures proper antigen presentation by an APC, to activate other immune cells. This is critical to rid the body of harmful infections.[13] For example, proper antigen presentation benefits T cell activation, and memory T cell survival and generation. Without it, T cells leaving their site of production and entering the circulatory vessels of the body will not be activated against a danger.[14] The immune system will not be able to kill dangerous or infected cells, and will not react quickly against a second infection.
MHC class II molecule stabilization - chaperonal function
The low pH of lysosomes could cause denaturation or proteolysis of MHC class II molecules. HLA-DM binding to MHC stabilizes and protects from degradation, by covering hydrophobic surfaces.[15] Antigen degradation could also ensue, resulting in an inability to bind to the peptide-binding groove. Thus, HLA-DM is needed to protect proteins against the lysosomal environment.[15]
CLIP release
In order to ensure that no false peptides bind to an MHC class II molecule, the peptide-binding groove is occupied by a protein called CLIP. Once a proper peptide is encountered, HLA-DM catalyzes the exchange of CLIP for an antigen peptide.[16] Often, this peptide is retrieved directly from the B cell receptor which internalized it. Through expulsion of CLIP at the proper time, HLA-DM ensures that the correct antigen can bind to MHC molecules and prevent either from degrading.[13]
Antigen loading and release
Apart from CLIP-antigen exchange, HLA-DM also facilitates antigen-antigen exchange. It releases weakly bound peptides from the groove to load peptides with higher-affinity binding. This process occurs in endosomes once they have left the ER containing MHC and HLA-DM that have fused with antigen-containing lysosomes.[16] Kinetic analysis studies have shown that HLA-DM loading occurs quickly and in many endosomes. Along the membrane of an endosome at the optimal acidity (pH=5.0), HLA-DM loads 3 to 12 peptides onto different MHC molecules per minute.[15]
HLA-DM assists in catalysis of peptide exchange not only in late endosomes traveling from the ER, but also on cell membranes and in early endosomes. Much of this pathway is still being researched, but it is known that HLA-DM can load exogenous peptides onto MHC class II molecules when they are being expressed on cell surfaces. Loading can also occur in early endosomes that are quickly recycled. In both of these areas, loading occurs slower due to an altered pH environment.[6]
Release
To release peptides from the MHC groove, HLA-DM binds to the N terminus of the groove, altering its conformation and breaking hydrogen bonds[2] such that the peptide that was interacting with the MHC groove can no longer bind and is ejected.[8]
Loading
Quick loading of peptides, facilitated by a stable MHC-DM complex, decreases the chances of those peptides being broken down by the proteolytic environment in the endosome.[11] HLA-DM dissociates from the MHC once a stable enough peptide has bound.[15] Thus, only antigens that can "outcompete" others by binding strongly enough to the groove end up on the surface of the antigen presenting cells in MHC class II molecules.[16]
Interaction with HLA-DO
HLA-DM also binds to HLA-DO, another non-classical MHC molecule. HLA-DO starts binding to DM in early endosomes, but is expressed less in late endosomes/lysosomes.[12] The binding between HLA-DM and HLA-DO is less strong at low pH, but overall much stronger than HLA-DM binding to MHC molecules.[14]
Before encountering an antigen, DO acts as a chaperone of DM to stabilize it against denaturation and direct it into lysosomes. It binds in the same location to HLA-DM as MHC class II molecules bind, thereby preventing HLA-DM from binding to MHC class II molecules. This inhibits peptide exchange catalysis and keeps CLIP in the MHC groove[16] until antigen-containing lysosome fuses with DM/DO/MHC containing lysosomes, prompting the degradation of HLA-DO molecules in MIICs.[14]
Structure and binding
HLA-DM contains a N-terminal class II histocompatibility antigen, alpha domain and a C-terminal Immunoglobulin C1-set domain.
Research in crystallography has resulted in advanced knowledge on HLA-DM structure, and how it binds to its substrates (HLA-DO and MHC class II molecules).[9]
HLA-DM Structure
The structure and sequence of HLA-DM proteins is very similar to other MHC class II molecules,[11] all of which consist of a heterodimer composed of an alpha and beta chain. However, HLA-DM differs in that it is nonclassical (meaning it lacks a transport signal N-terminus), and does not have the capability to bind peptides. This is due to lack of a deep peptide binding groove – instead, it contains a shallow, negatively charged indent with two disulfide bonds.[5]
On its beta chain cytoplasmic tail, a tyrosine-based motif YTPL regulates trafficking to specific endosomal compartments called MHC class II compartments (MIICs) from the ER.[2]
Binding with MHC class II
HLA-DM catalyzes peptide exchange through binding at the beta chain of MHC class II molecules,[16] which alters the conformation of the MHC and its peptide-binding groove. HLA-DM conformation stays constant.[17] When a peptide is bound to the P1 locus in the peptide binding groove, it is stably bound. This also hinders HLA-DM binding to the MHC, preventing destabilization of the peptide-MHC interaction.[12] Peptides also bind to the C-terminal site of the binding groove, but in this case the binding is a weak association, leaving the N-terminal of the groove open. HLA-DM can then bind to the N-terminal and allowing for peptide exchange.[12]
Binding with HLA-DO
HLA-DO binds to the same regions of HLA-DM as MHC class II molecules do, such that it blocks the ability of HLA-DM to bind with MHC.[12] Thus, you can never have a complex containing HLA-DM, HLA-DO, and MHC class II molecules.
Expression and Location
Intracellularly, HLA-DM is translated in the endoplasmic reticulum, then transported to endosomal MHC class II compartments (MIICs). MIICs then join with endosomes containing MHC class II molecules bound to CLIP. Here, the HLA-DM begins editing the MHC peptide binding.[2]
HLA-DM is also expressed on the surface of B cells and dendritic cells,[6] as well as in secreted exosomes.[18]
During B cell development, HLA-DM is first expressed in early stages in the bone marrow. Expression then remains high throughout development and a B cell’s life, until the B cell differentiates into a plasma cell and HLA-DM expression then decreases.[14]
Within the body, highest levels of HLA-DM expression is found in lymph nodes, the spleen, and bone marrow.[4]
Role in Disease and Medicine
Immunodeficiency
In individuals lacking functional HLA-DM molecules, improper antigen presentation occurs, resulting in unwanted immune responses or lack of a response when danger is present.[8] This has been shown experimentally through mouse knockout models.[5] There will be an increase of CLIP, instead of peptide, presentation on APC surfaces. This can result in autoimmunity, if a T cell receptors recognize CLIP as a harmful antigen. There could also be no protein presentation at all, resulting in a lack of immune response.[8]
Infections and Disease
Type 1 diabetes is correlated with DM activation, which is hypothesized to be due to DM positively modulating the expression of disease-causing peptides in the MHC groove and thus presented to responding T cells.[12] Experiments using the mouse model of type 1 diabetes which blocked DM or reduced its activity by overexpressing DO found a decrease in diabetes.[12]
HLA-DM is implicated in viral infections like Herpes Simplex Virus Type 1. This virus causes uneven distribution of HLA-DM in endosomes, prevents peptide catalysis, and prevents presentation of MHC class II molecules on the cell surface.[2]
HLA-DM is also implicated in celiac disease, multiple sclerosis, other autoimmune diseases, and leukemia.[6][19][20]
References
- PDB: 2BC4; Nicholson MJ, Moradi B, Seth NP, Xing X, Cuny GD, Stein RL, Wucherpfennig KW (April 2006). "Small molecules that enhance the catalytic efficiency of HLA-DM". Journal of Immunology. 176 (7): 4208–20. doi:10.4049/jimmunol.176.7.4208. PMC 3412064. PMID 16547258.
- McCracken R. "HLA-DM". www.bio.davidson.edu. Retrieved 2018-01-29.
- Busch R, Rinderknecht CH, Roh S, Lee AW, Harding JJ, Burster T, Hornell TM, Mellins ED (October 2005). "Achieving stability through editing and chaperoning: regulation of MHC class II peptide binding and expression". Immunological Reviews. 207: 242–60. doi:10.1111/j.0105-2896.2005.00306.x. PMID 16181341. S2CID 24207944.
- "HLA-DMA major histocompatibility complex, class II, DM alpha [Homo sapiens (human)] - Gene - NCBI". www.ncbi.nlm.nih.gov. Retrieved 2018-03-06.
- Vogt AB, Kropshofer H (April 1999). "HLA-DM - an endosomal and lysosomal chaperone for the immune system". Trends in Biochemical Sciences. 24 (4): 150–4. doi:10.1016/S0968-0004(99)01364-X. PMID 10322421.
- Arndt SO, Vogt AB, Markovic-Plese S, Martin R, Moldenhauer G, Wölpl A, Sun Y, Schadendorf D, Hämmerling GJ, Kropshofer H (March 2000). "Functional HLA-DM on the surface of B cells and immature dendritic cells". The EMBO Journal. 19 (6): 1241–51. doi:10.1093/emboj/19.6.1241. PMC 305665. PMID 10716924.
- Pashine A, Busch R, Belmares MP, Munning JN, Doebele RC, Buckingham M, Nolan GP, Mellins ED (August 2003). "Interaction of HLA-DR with an acidic face of HLA-DM disrupts sequence-dependent interactions with peptides". Immunity. 19 (2): 183–92. doi:10.1016/S1074-7613(03)00200-0. PMID 12932352.
- Yin L, Maben ZJ, Becerra A, Stern LJ (July 2015). "Evaluating the Role of HLA-DM in MHC Class II-Peptide Association Reactions". Journal of Immunology. 195 (2): 706–16. doi:10.4049/jimmunol.1403190. PMC 4490944. PMID 26062997.
- Mosyak L, Zaller DM, Wiley DC (September 1998). "The structure of HLA-DM, the peptide exchange catalyst that loads antigen onto class II MHC molecules during antigen presentation". Immunity. 9 (3): 377–83. doi:10.1016/s1074-7613(00)80620-2. PMID 9768757.
- Berczi I, Szentivanyi A (2003). "Antigen presentation". NeuroImmune Biology. Vol. 3. pp. 301–313. doi:10.1016/s1567-7443(03)80053-4. ISBN 9780444508515.
- Schulze MS, Wucherpfennig KW (February 2012). "The mechanism of HLA-DM induced peptide exchange in the MHC class II antigen presentation pathway". Current Opinion in Immunology. 24 (1): 105–11. doi:10.1016/j.coi.2011.11.004. PMC 3288754. PMID 22138314.
- Mellins ED, Stern LJ (February 2014). "HLA-DM and HLA-DO, key regulators of MHC-II processing and presentation". Current Opinion in Immunology. 26: 115–22. doi:10.1016/j.coi.2013.11.005. PMC 3944065. PMID 24463216.
- Fundamental immunology. Paul, William E. (7th ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. 2013. ISBN 978-1-4511-1783-7. OCLC 856655285.
{{cite book}}: CS1 maint: others (link) - Adler LN, Jiang W, Bhamidipati K, Millican M, Macaubas C, Hung SC, Mellins ED (2017). "The Other Function: Class II-Restricted Antigen Presentation by B Cells". Frontiers in Immunology. 8: 319. doi:10.3389/fimmu.2017.00319. PMC 5362600. PMID 28386257.
- Vogt AB, Kropshofer H (April 1999). "HLA-DM - an endosomal and lysosomal chaperone for the immune system". Trends in Biochemical Sciences. 24 (4): 150–4. doi:10.1016/s0968-0004(99)01364-x. PMID 10322421.
- A., Owen, Judith (2013). Kuby immunology. Punt, Jenni., Stranford, Sharon A., Jones, Patricia P., Kuby, Janis. (7th ed.). New York: W.H. Freeman. ISBN 9781464119910. OCLC 820117219.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Yin L, Trenh P, Guce A, Wieczorek M, Lange S, Sticht J, Jiang W, Bylsma M, Mellins ED, Freund C, Stern LJ (August 2014). "Susceptibility to HLA-DM protein is determined by a dynamic conformation of major histocompatibility complex class II molecule bound with peptide". The Journal of Biological Chemistry. 289 (34): 23449–64. doi:10.1074/jbc.m114.585539. PMC 4156084. PMID 25002586.
- Xiu F, Côté MH, Bourgeois-Daigneault MC, Brunet A, Gauvreau MÉ, Shaw A, Thibodeau J (August 2011). "Cutting edge: HLA-DO impairs the incorporation of HLA-DM into exosomes". Journal of Immunology. 187 (4): 1547–51. doi:10.4049/jimmunol.1100199. PMID 21768396.
- Wang J, Song D, Liu Y, Lu G, Yang S, Liu L, Gao Z, Ma L, Guo Z, Zhang C, Wang H, Yang B (October 2017). "HLA-DMB restricts human T-cell leukemia virus type-1 (HTLV-1) protein expression via regulation of ATG7 acetylation". Scientific Reports. 7 (1): 14416. Bibcode:2017NatSR...714416W. doi:10.1038/s41598-017-14882-z. PMC 5663917. PMID 29089548.
- Pietz G, De R, Hedberg M, Sjöberg V, Sandström O, Hernell O, Hammarström S, Hammarström ML (2017-09-21). "Immunopathology of childhood celiac disease-Key role of intestinal epithelial cells". PLOS ONE. 12 (9): e0185025. Bibcode:2017PLoSO..1285025P. doi:10.1371/journal.pone.0185025. PMC 5608296. PMID 28934294.
External links
- Overview at Davidson College (student generated)
- Protein Data Bank