Methyl fluorosulfonate
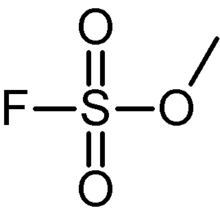
Methyl fluorosulfonate, also known as magic methyl, is the organic compound with the formula FSO2OCH3. It is a colorless liquid that is used as a strong methylating agent in organic synthesis. Because of its extreme toxicity, it has largely been replaced by the related reagent methyl trifluoromethanesulfonate.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Methyl sulfurofluoridate | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.006.369 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| CH3FO3S | |
| Molar mass | 114.09 g·mol−1 |
| Density | 1.45 g/mL |
| Boiling point | 93 °C (199 °F; 366 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Synthesis and reactions
It is prepared by distillation of an equimolar mixture of fluorosulfonic acid and dimethyl sulfate. It was originally produced by the reaction of methanol with fluorosulfonic acid.[1]
Methyl fluorosulfonate is a highly electrophilic reagent for methylation. It is ranked as less powerful than methyl trifluoromethanesulfonate.[2]
Toxicity
Similar to phosgene, it is acutely toxic[1] by inhalation, with an LC50 (rat, 1 hour) of about 5 ppm. Several cases of poisoning resulting in death from pulmonary edema have been reported.[3]
References
- Meyer, Julius; Schramm, Georg (1932). "Ester der Fluorsulfonsäure (Esters of fluorosulfonic acid)". Zeitschrift für Anorganische und Allgemeine Chemie. 206: 24–30. doi:10.1002/zaac.19322060103.
- Stang, Peter J.; Hanack, Michael; Subramanian, L. R. (1982). "Perfluoroalkanesulfonic Esters: Methods of Preparation and Applications in Organic Chemistry". Synthesis. 1982 (2): 85–126. doi:10.1055/s-1982-29711. ISSN 0039-7881.
- Hite, M.; Rinehart, W.; Braun, W.; Peck, H. (1979). "Acute toxicity of methyl fluorosulfonate (Magic Methyl)". AIHA Journal. 40 (7): 600–603. doi:10.1080/00028897708984416. PMID 484483.