Magnetofection
Magnetofection is a transfection method that uses magnetic fields to concentrate particles containing vectors to target cells in the body.[1] Magnetofection has been adapted to a variety of vectors, including nucleic acids, non-viral transfection systems, and viruses. This method offers advantages such as high transfection efficiency and biocompatibility which are balanced with limitations.
Mechanism
Principle
The term magnetofection, currently trademarked by the company OZ Biosciences, combines the words magnetic and transfection.[2] Magnetofection uses nucleic acids associated with magnetic nanoparticles. These molecular complexes are then concentrated and transported into cells using an applied magnetic field.
Synthesis
The magnetic nanoparticles are typically made from iron oxide, which is fully biodegradable, using methods such as coprecipitation or microemulsion.[3][4]
The nanoparticles are then combined with gene vectors (DNA, siRNA, ODN, virus, etc.). One method involves linking viral particles to magnetic particles using an avidin-biotin interaction.[5] Viruses can also bind to the nanoparticles via hydrophobic interaction.[6]
Another synthesis method involves coating magnetic nanoparticles with cationic lipids or polymers via salt-induced aggregation. For example, nanoparticles may be conjugated with the polyethylenimine (PEI), a positively charged polymer used commonly as a transfection agent.[7] The PEI solution must have a high pH during synthesis to encourage high gene expression.[8] The positively charged nanoparticles can then associate with negatively charged nucleic acids via electrostatic interaction.[9]
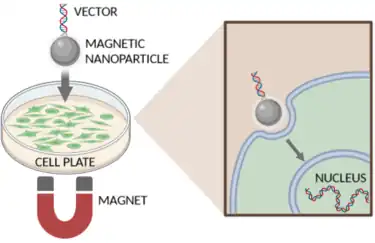
Cellular uptake
Magnetic particles loaded with vectors are concentrated on the target cells by the influence of an external magnetic field. The cells then take up genetic material naturally via endocytosis and pinocytosis. Consequently, membrane architecture and structure stays intact, in contrast to other physical transfection methods such as electroporation or gene guns that damage the cell membrane.[10]
The nucleic acids are then released into the cytoplasm by different mechanisms depending upon the formulation used:
- the proton sponge effect caused by cationic polymers coated on the nanoparticles that promote endosome osmotic swelling, disruption of the endosome membrane and intracellular release of DNA form,
- the destabilization of endosome by cationic lipids coated on the particles that release the nucleic acid into cells by flip-flop of cell negative lipids and charge neutralization and
- the viral infection mechanism.
Magnetofection works with cells that are not dividing or slowly dividing, meaning that the genetic materials can go to the cell nucleus without cell division.[11]
Applications
Magnetofection has been tested on a broad range of cell lines, hard-to-transfect and primary cells.[12] Several optimized and efficient magnetic nanoparticle formulations have been specifically developed for several types of applications such as DNA, siRNA, and primary neuron transfection as well as viral applications.[13]
Magnetofection research is currently in the preclinical stage. This technique has primarily been tested in vivo using plasmid DNA in mouse, rat, and rabbit models for applications in the hippocampus, subcutaneous tumors, lungs, spinal cord, and muscle.[14]
Some applications include:
- Delivery of GFP gene into primary neural stem cells, which are typically difficult to transfect, with 18% efficacy with a static magnetic field and 32% efficacy with an oscillating field.[15]
- Delivery of oligodesoxynucleotides (ODN) into human umbilical vein endothelial cells with 84% efficiency.[16]
- Delivery of siRNA to HeLa cells to knock down luciferase reporter gene.[17]
- Delivery of adenoviral vectors to primary human peripheral blood lymphocytes.[18]
Advantages
Magnetofection attempts to unite the advantages of biochemical (cationic lipids or polymers) and physical (electroporation, gene gun) transfection methods. It allows for local delivery with high transfection efficiency, faster incubation time, and biocompatibility.[19]
Transfection efficiency
Coupling magnetic nanoparticles to gene vectors results in hundreds-fold increase of the uptake of these vectors on a time scale of minutes, thus leading to high transfection efficiency.[20] Gene vector and magnetic nanoparticle complexes are transfected into cells after 10–15 minutes, which is faster than the 2–4 hours that other transfection methods require.[21] After 24, 48 or 72 hours, most of the particles are localized in the cytoplasm, in vacuoles (membranes surrounded structure into cells) and occasionally in the cell nucleus.[22]
Biocompatibility
Magnetic nanoparticles do not aggregate easily once the magnet is removed, and therefore are unlikely to block capillaries or cause thrombosis.[23] In addition, iron oxide is biodegradable, and the iron can be reused in hemoglobin or iron metabolism pathways.[24][25]
Disadvantages
Particle variability
Magnetic nanoparticle synthesis can sometimes lead to a wide range of differently sized particles.[26] The size of particles can influence their usefulness. Specifically, nanoparticles that are less than 10 nm or greater than 200 nm in size tend to be cleared from the body more quickly.[27]
Localization in vivo
While magnets can be used to localize magnetic nanoparticles to desired cells, this mechanism may be difficult to maintain in practice. The nanoparticles can be concentrated in 2D space such as on a culture plate or at the surface of the body, but it can be more difficult to localize them in the 3D space of the body. Magnetofection does not work well for organs or blood vessels far from the surface of the body, since the magnetic field weakens as distance increases.[28][29] In addition, the user must consider the frequency and timing of applying the magnetic field, as the particles will not necessarily stay in the desired location once the magnet is removed.[30]
Cytotoxicity
While iron oxide used to make nanoparticles is biodegradable, the toxicity of magnetic nanoparticles is still under investigation. Some research has found no signs of damage to cells, while others claim that small (< 2 nm) nanoparticles can diffuse across cell membranes and disrupt organelles.[31][32]
In addition, very high concentrations of iron oxide can disrupt homeostasis and lead to iron overload, which can damage or alter DNA, affect cellular responses, and kill cells.[33] Lysosymes can also digest the nanoparticles and release free iron which can react with hydrogen peroxide to form free radicals, leading to cytotoxic, mutagenic, and carcinogenic effects.[34]
References
- Plank C, Zelphati O, Mykhaylyk O (November 2011). "Magnetically enhanced nucleic acid delivery. Ten years of magnetofection-progress and prospects". Advanced Drug Delivery Reviews. 63 (14–15): 1300–1331. doi:10.1016/j.addr.2011.08.002. PMC 7103316. PMID 21893135.
- "MAGNETOFECTION Trademark of OZ BIOSCIENCES - Registration Number 5116540 - Serial Number 79182650 :: Justia Trademarks". trademarks.justia.com. Retrieved 2021-11-19.
- Arbab AS, Bashaw LA, Miller BR, Jordan EK, Lewis BK, Kalish H, Frank JA (December 2003). "Characterization of biophysical and metabolic properties of cells labeled with superparamagnetic iron oxide nanoparticles and transfection agent for cellular MR imaging". Radiology. 229 (3): 838–846. doi:10.1148/radiol.2293021215. PMID 14657318.
- Majidi S, Sehrig FZ, Farkhani SM, Goloujeh MS, Akbarzadeh A (2016-02-17). "Current methods for synthesis of magnetic nanoparticles". Artificial Cells, Nanomedicine, and Biotechnology. 44 (2): 722–734. doi:10.3109/21691401.2014.982802. PMID 25435409. S2CID 31332211.
- Mah C, Fraites TJ, Zolotukhin I, Song S, Flotte TR, Dobson J, et al. (July 2002). "Improved method of recombinant AAV2 delivery for systemic targeted gene therapy". Molecular Therapy. 6 (1): 106–112. doi:10.1006/mthe.2001.0636. PMID 12095310.
- Agopian K, Wei BL, Garcia JV, Gabuzda D (March 2006). "A hydrophobic binding surface on the human immunodeficiency virus type 1 Nef core is critical for association with p21-activated kinase 2". Journal of Virology. 80 (6): 3050–3061. doi:10.1128/jvi.80.6.3050-3061.2006. PMC 1395437. PMID 16501114.
- Cui Y, Li X, Zeljic K, Shan S, Qiu Z, Wang Z (October 2019). "Effect of PEGylated Magnetic PLGA-PEI Nanoparticles on Primary Hippocampal Neurons: Reduced Nanoneurotoxicity and Enhanced Transfection Efficiency with Magnetofection". ACS Applied Materials & Interfaces. 11 (41): 38190–38204. doi:10.1021/acsami.9b15014. PMID 31550131. S2CID 202762218.
- Prosen, Lara; Prijic, Sara; Music, Branka; Lavrencak, Jaka; Cemazar, Maja; Sersa, Gregor (2013-06-03). "Magnetofection: A Reproducible Method for Gene Delivery to Melanoma Cells". BioMed Research International. 2013: e209452. doi:10.1155/2013/209452. ISSN 2314-6133. PMC 3686069. PMID 23862136.
- Scherer F, Anton M, Schillinger U, Henke J, Bergemann C, Krüger A, et al. (January 2002). "Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo". Gene Therapy. 9 (2): 102–109. doi:10.1038/sj.gt.3301624. PMID 11857068. S2CID 1565485.
- Fus-Kujawa, Agnieszka; Prus, Pawel; Bajdak-Rusinek, Karolina; Teper, Paulina; Gawron, Katarzyna; Kowalczuk, Agnieszka; Sieron, Aleksander L. (2021-07-20). "An Overview of Methods and Tools for Transfection of Eukaryotic Cells in vitro". Frontiers in Bioengineering and Biotechnology. 9: 701031. doi:10.3389/fbioe.2021.701031. ISSN 2296-4185. PMC 8330802. PMID 34354988.
- Fajrial, Apresio K.; He, Qing Qing; Wirusanti, Nurul I.; Slansky, Jill E.; Ding, Xiaoyun (2020). "A review of emerging physical transfection methods for CRISPR/Cas9-mediated gene editing". Theranostics. 10 (12): 5532–5549. doi:10.7150/thno.43465. ISSN 1838-7640. PMC 7196308. PMID 32373229.
- Plank C, Anton M, Rudolph C, Rosenecker J, Krötz F (August 2003). "Enhancing and targeting nucleic acid delivery by magnetic force". Expert Opinion on Biological Therapy. 3 (5): 745–758. doi:10.1517/14712598.3.5.745. PMID 12880375. S2CID 865185.
- Plank, Christian; Zelphati, Olivier; Mykhaylyk, Olga (2011-11-01). "Magnetically enhanced nucleic acid delivery. Ten years of magnetofection—Progress and prospects". Advanced Drug Delivery Reviews. Hybrid nanostructures for diagnostics and therapeutics. 63 (14): 1300–1331. doi:10.1016/j.addr.2011.08.002. ISSN 0169-409X. PMC 7103316. PMID 21893135.
- Sizikov AA, Kharlamova MV, Nikitin MP, Nikitin PI, Kolychev EL (April 2021). "Nonviral Locally Injected Magnetic Vectors for In Vivo Gene Delivery: A Review of Studies on Magnetofection". Nanomaterials. 11 (5): 1078. doi:10.3390/nano11051078. PMC 8143545. PMID 33922066.
- Pickard, Mark R.; Adams, Christopher F.; Barraud, Perrine; Chari, Divya M. (2015). "Using Magnetic Nanoparticles for Gene Transfer to Neural Stem Cells: Stem Cell Propagation Method Influences Outcomes". Journal of Functional Biomaterials. 6 (2): 259–276. doi:10.3390/jfb6020259. PMC 4493511. PMID 25918990.
- Krötz, Florian; Wit, Cor de; Sohn, Hae-Young; Zahler, Stefan; Gloe, Torsten; Pohl, Ulrich; Plank, Christian (2003-05-01). "Magnetofection—A highly efficient tool for antisense oligonucleotide delivery in vitro and in vivo". Molecular Therapy. 7 (5): 700–710. doi:10.1016/S1525-0016(03)00065-0. ISSN 1525-0016. PMID 12718913.
- Schillinger, Ulrike (2005). "Advances in magnetofection—magnetically guided nucleic acid delivery". Journal of Magnetism and Magnetic Materials. 293 (1): 501–508. Bibcode:2005JMMM..293..501S. doi:10.1016/j.jmmm.2005.01.032. S2CID 122076397 – via Elsivier.
- Scherer, F.; Anton, M.; Schillinger, U.; Henke, J.; Bergemann, C.; Krüger, A.; Gänsbacher, B.; Plank, C. (2002). "Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo". Gene Therapy. 9 (2): 102–109. doi:10.1038/sj.gt.3301624. ISSN 1476-5462. PMID 11857068. S2CID 1565485.
- Plank, Christian; Zelphati, Olivier; Mykhaylyk, Olga (2011). "Magnetically enhanced nucleic acid delivery. Ten years of magnetofection—Progress and prospects". Advanced Drug Delivery Reviews. 63 (14): 1300–1331. doi:10.1016/j.addr.2011.08.002. ISSN 0169-409X. PMC 7103316. PMID 21893135.
- Scherer, F.; Anton, M.; Schillinger, U.; Henke, J.; Bergemann, C.; Krüger, A.; Gänsbacher, B.; Plank, C. (2002). "Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo". Gene Therapy. 9 (2): 102–109. doi:10.1038/sj.gt.3301624. ISSN 1476-5462. PMID 11857068. S2CID 1565485.
- Scherer, F.; Anton, M.; Schillinger, U.; Henke, J.; Bergemann, C.; Krüger, A.; Gänsbacher, B.; Plank, C. (2002). "Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo". Gene Therapy. 9 (2): 102–109. doi:10.1038/sj.gt.3301624. ISSN 1476-5462. PMID 11857068. S2CID 1565485.
- Santori MI, Gonzalez C, Serrano L, Isalan M (2006-06-27). "Localized transfection with magnetic beads coated with PCR products and other nucleic acids". Nature Protocols. 1 (2): 526–531. doi:10.1038/nprot.2006.74. PMID 17406278. S2CID 23641355.
- Wahajuddin; Arora, Sumit (2012). "Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers". International Journal of Nanomedicine. 7: 3445–3471. doi:10.2147/IJN.S30320. ISSN 1176-9114. PMC 3405876. PMID 22848170.
- Castillo, Betzaida; Bromberg, Lev; López, Xaira; Badillo, Valerie; González Feliciano, Jose A.; González, Carlos I.; Hatton, T. Alan; Barletta, Gabriel (2012-08-30). "Intracellular Delivery of siRNA by Polycationic Superparamagnetic Nanoparticles". Journal of Drug Delivery. 2012: e218940. doi:10.1155/2012/218940. ISSN 2090-3014. PMC 3437298. PMID 22970377.
- Wahajuddin; Arora, Sumit (2012). "Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers". International Journal of Nanomedicine. 7: 3445–3471. doi:10.2147/IJN.S30320. ISSN 1176-9114. PMC 3405876. PMID 22848170.
- Prosen, Lara; Prijic, Sara; Music, Branka; Lavrencak, Jaka; Cemazar, Maja; Sersa, Gregor (2013-06-03). "Magnetofection: A Reproducible Method for Gene Delivery to Melanoma Cells". BioMed Research International. 2013: e209452. doi:10.1155/2013/209452. ISSN 2314-6133. PMC 3686069. PMID 23862136.
- Yetisgin, Abuzer Alp; Cetinel, Sibel; Zuvin, Merve; Kosar, Ali; Kutlu, Ozlem (2020). "Therapeutic Nanoparticles and Their Targeted Delivery Applications". Molecules. 25 (9): 2193. doi:10.3390/molecules25092193. PMC 7248934. PMID 32397080.
- Wahajuddin; Arora, Sumit (2012). "Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers". International Journal of Nanomedicine. 7: 3445–3471. doi:10.2147/IJN.S30320. ISSN 1176-9114. PMC 3405876. PMID 22848170.
- Mahmoudi, Morteza; Sant, Shilpa; Wang, Ben; Laurent, Sophie; Sen, Tapas (2011-01-01). "Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy". Advanced Drug Delivery Reviews. 2011 Editors' Collection. 63 (1): 24–46. doi:10.1016/j.addr.2010.05.006. ISSN 0169-409X. PMID 20685224.
- Schneider-Futschik, Elena K.; Reyes-Ortega, Felisa (2021). "Advantages and Disadvantages of Using Magnetic Nanoparticles for the Treatment of Complicated Ocular Disorders". Pharmaceutics. 13 (8): 1157. doi:10.3390/pharmaceutics13081157. PMC 8400382. PMID 34452117.
- Wei, Hao; Hu (2021). "Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers". International Journal of Nanomedicine. 7: 3445–3471. doi:10.2147/IJN.S30320. ISSN 1178-2013. PMC 3405876. PMID 22848170.
- Wahajuddin; Arora, Sumit (2012-07-06). "Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers". International Journal of Nanomedicine. 7: 3445–3471. doi:10.2147/IJN.S30320. PMC 3405876. PMID 22848170.
- Valdiglesias, Vanessa; Kiliç, Gözde; Costa, Carla; Fernández-Bertólez, Natalia; Pásaro, Eduardo; Teixeira, João Paulo; Laffon, Blanca (2015). "Effects of iron oxide nanoparticles: Cytotoxicity, genotoxicity, developmental toxicity, and neurotoxicity". Environmental and Molecular Mutagenesis. 56 (2): 125–148. doi:10.1002/em.21909. ISSN 1098-2280. PMID 25209650. S2CID 46117152.
- Toyokuni, Shinya (1996-01-01). "Iron-induced carcinogenesis: The role of redox regulation". Free Radical Biology and Medicine. 20 (4): 553–566. doi:10.1016/0891-5849(95)02111-6. ISSN 0891-5849. PMID 8904296.
Further reading
- Mair L, Ford K, Alam M, Kole R, Fisher M, Superfine R (April 2009). "Size-uniform 200 nm particles: fabrication and application to magnetofection". Journal of Biomedical Nanotechnology. 5 (2): 182–191. doi:10.1166/jbn.2009.1024. PMC 2818021. PMID 20055096.