Diabetes management
The term diabetes includes several different metabolic disorders that all, if left untreated, result in abnormally high concentrations of a sugar called glucose in the blood. Diabetes mellitus type 1 results when the pancreas no longer produces significant amounts of the hormone insulin, usually owing to the autoimmune destruction of the insulin-producing beta cells of the pancreas. Diabetes mellitus type 2, in contrast, is now thought to result from autoimmune attacks on the pancreas and/or insulin resistance. The pancreas of a person with type 2 diabetes may be producing normal or even abnormally large amounts of insulin. Other forms of diabetes mellitus, such as the various forms of maturity-onset diabetes of the young, may represent some combination of insufficient insulin production and insulin resistance. Some degree of insulin resistance may also be present in a person with type 1 diabetes.
The main goal of diabetes management and control is, as far as possible, to restore carbohydrate metabolism to a normal state. To achieve this goal, individuals with an absolute deficiency of insulin require insulin replacement therapy, which is given through injections or an insulin pump. Insulin resistance, in contrast, can be corrected by dietary modifications and exercise. Other goals of diabetes management are to prevent or treat the many complications that can result from the disease itself and from its treatment.[1]
Overview
Goals
The treatment goals are related to effective control of blood glucose, blood pressure and lipids, to minimize the risk of long-term consequences associated with diabetes. They are suggested in clinical practice guidelines released by various national and international diabetes agencies.[2][3]
The targets are:
- HbA1c of less than 6% or 7.0% if they are achievable without significant hypoglycemia[4][5]
- Preprandial blood glucose: 3.9 to 7.2 mmol/L (70 to 130 mg/dL)[4]
- 2-hour postprandial blood glucose: <10 mmol/L (<180 mg/dL)[4]
Goals should be individualized based on:[4]
- Duration of diabetes
- Age/life expectancy
- Comorbidity
- Known cardiovascular disease or advanced microvascular disease
- Hypoglycemia awareness
In older patients, clinical practice guidelines by the American Geriatrics Society state "for frail older adults, persons with life expectancy of less than 5 years, and others in whom the risks of intensive glycemic control appear to outweigh the benefits, a less stringent target such as HbA1c of 8% is appropriate".[6]
Issues
The primary issue requiring management is that of the glucose cycle. In this, glucose in the bloodstream is made available to cells in the body; a process dependent upon the twin cycles of glucose entering the bloodstream, and insulin allowing appropriate uptake into the body cells. Both aspects can require management. Another issue that ties along with the glucose cycle is getting a balanced amount of the glucose to the major organs so they are not affected negatively.
Complexities

The main complexities stem from the nature of the feedback loop of the glucose cycle, which is sought to be regulated:
- The glucose cycle is a system which is affected by two factors: entry of glucose into the bloodstream and also blood levels of insulin to control its transport out of the bloodstream
- As a system, it is sensitive to diet and exercise
- It is affected by the need for user anticipation due to the complicating effects of time delays between any activity and the respective impact on the glucose system
- Management is highly intrusive, and compliance is an issue, since it relies upon user lifestyle change and often upon regular sampling and measuring of blood glucose levels, multiple times a day in many cases
- It changes as people grow and develop
- It is highly individual
As diabetes is a prime risk factor for cardiovascular disease, controlling other risk factors which may give rise to secondary conditions, as well as the diabetes itself, is one of the facets of diabetes management. Checking cholesterol, LDL, HDL and triglyceride levels may indicate hyperlipoproteinemia, which may warrant treatment with hypolipidemic drugs. Checking the blood pressure and keeping it within strict limits (using diet and antihypertensive treatment) protects against the retinal, renal and cardiovascular complications of diabetes. Regular follow-up by a podiatrist or other foot health specialists is encouraged to prevent the development of diabetic foot. Annual eye exams are suggested to monitor for progression of diabetic retinopathy.
Early advancements
Late in the 19th century, sugar in the urine (glycosuria) was associated with diabetes. Various doctors studied the connection. Frederick Madison Allen studied diabetes in 1909–12, then published a large volume, Studies Concerning Glycosuria and Diabetes, (Boston, 1913). He invented a fasting treatment for diabetes called the Allen treatment for diabetes. His diet was an early attempt at managing diabetes.
Blood sugar level
Blood sugar level is measured by means of a glucose meter, with the result either in mg/dL (milligrams per deciliter in the US) or mmol/L (millimoles per litre in Canada and Eastern Europe) of blood. The average normal person has an average fasting glucose level of 4.5 mmol/L (81 mg/dL), with a lows of down to 2.5 and up to 5.4 mmol/L (65 to 98 mg/dL).[7]
Optimal management of diabetes involves patients measuring and recording their own blood glucose levels. By keeping a diary of their own blood glucose measurements and noting the effect of food and exercise, patients can modify their lifestyle to better control their diabetes. For patients on insulin, patient involvement is important in achieving effective dosing and timing.
Hypo and hyperglycemia
Levels which are significantly above or below this range are problematic and can in some cases be dangerous. A level of <3.8 mmol/L (<70 mg/dL) is usually described as a hypoglycemic attack (low blood sugar). Most diabetics know when they are going to "go hypo" and usually are able to eat food or drink something sweet to raise their levels. A patient who is hyperglycemic (high glucose) can also become temporarily hypoglycemic under certain conditions (e.g. not eating regularly, or after strenuous exercise, followed by fatigue). Intensive efforts to achieve blood sugar levels close to normal have been shown to triple the risk of the most severe form of hypoglycemia, in which the patient requires assistance from by-standers in order to treat the episode.[8] In the United States, there were annually 48,500 hospitalizations for diabetic hypoglycemia and 13,100 for diabetic hypoglycemia resulting in coma in the period 1989 to 1991, before intensive blood sugar control was as widely recommended as today.[9] One study found that hospital admissions for diabetic hypoglycemia increased by 50% from 1990–1993 to 1997–2000, as strict blood sugar control efforts became more common.[10] Among intensively controlled type 1 diabetics, 55% of episodes of severe hypoglycemia occur during sleep, and 6% of all deaths in diabetics under the age of 40 are from nocturnal hypoglycemia in the so-called 'dead-in-bed syndrome,' while National Institute of Health statistics show that 2% to 4% of all deaths in diabetics are from hypoglycemia.[11] In children and adolescents following intensive blood sugar control, 21% of hypoglycemic episodes occurred without explanation.[12] In addition to the deaths caused by diabetic hypoglycemia, periods of severe low blood sugar can also cause permanent brain damage.[13] Although diabetic nerve disease is usually associated with hyperglycemia, hypoglycemia as well can initiate or worsen neuropathy in diabetics intensively struggling to reduce their hyperglycemia.[14]
Levels greater than 13–15 mmol/L (230–270 mg/dL) are considered high, and should be monitored closely to ensure that they reduce rather than continue to remain high. The patient is advised to seek urgent medical attention as soon as possible if blood sugar levels continue to rise after 2–3 tests. High blood sugar levels are known as hyperglycemia, which is not as easy to detect as hypoglycemia and usually happens over a period of days rather than hours or minutes. If left untreated, this can result in diabetic coma and death.

Prolonged and elevated levels of glucose in the blood, which is left unchecked and untreated, will, over time, result in serious diabetic complications in those susceptible and sometimes even death. There is currently no way of testing for susceptibility to complications. Diabetics are therefore recommended to check their blood sugar levels either daily or every few days. There is also diabetes management software available from blood testing manufacturers which can display results and trends over time. Type 1 diabetics normally check more often, due to insulin therapy.
A history of blood sugar level results is especially useful for the diabetic to present to their doctor or physician in the monitoring and control of the disease. Failure to maintain a strict regimen of testing can accelerate symptoms of the condition, and it is therefore imperative that any diabetic patient strictly monitor their glucose levels regularly.
Glycemic control
Glycemic control is a medical term referring to the typical levels of blood sugar (glucose) in a person with diabetes mellitus. Much evidence suggests that many of the long-term complications of diabetes, especially the microvascular complications, result from many years of hyperglycemia (elevated levels of glucose in the blood). Good glycemic control, in the sense of a "target" for treatment, has become an important goal of diabetes care, although recent research suggests that the complications of diabetes may be caused by genetic factors[15][16] or, in type 1 diabetics, by the continuing effects of the autoimmune disease which first caused the pancreas to lose its insulin-producing ability.[17]
Because blood sugar levels fluctuate throughout the day and glucose records are imperfect indicators of these changes, the percentage of hemoglobin which is glycated is used as a proxy measure of long-term glycemic control in research trials and clinical care of people with diabetes. This test, the hemoglobin A1c or glycated hemoglobin reflects average glucose levels over the preceding 2–3 months. In nondiabetic persons with normal glucose metabolism the glycated hemoglobin is usually 4–6% by the most common methods (normal ranges may vary by method).
"Perfect glycemic control" would mean that glucose levels were always normal (70–130 mg/dL, or 3.9–7.2 mmol/L) and indistinguishable from a person without diabetes. In reality, because of the imperfections of treatment measures, even "good glycemic control" describes blood glucose levels that average somewhat higher than normal much of the time. In addition, one survey of type 2 diabetics found that they rated the harm to their quality of life from intensive interventions to control their blood sugar to be just as severe as the harm resulting from intermediate levels of diabetic complications.[18]
In the 1990s the American Diabetes Association conducted a publicity campaign to persuade patients and physicians to strive for average glucose and hemoglobin A1c values below 200 mg/dL (11 mmol/L) and 8%. Currently, many patients and physicians attempt to do better than that.
As of 2015 the guidelines called for an HbA1c of around 7% or a fasting glucose of less than 7.2 mmol/L (130 mg/dL); however these goals may be changed after professional clinical consultation, taking into account particular risks of hypoglycemia and life expectancy.[19][20] Despite guidelines recommending that intensive blood sugar control be based on balancing immediate harms and long-term benefits, many people – for example people with a life expectancy of less than nine years – who will not benefit are over-treated and do not experience clinically meaningful benefits.[21]
Poor glycemic control refers to persistently elevated blood glucose and glycated hemoglobin levels, which may range from 200 to 500 mg/dL (11–28 mmol/L) and 9–15% or higher over months and years before severe complications occur. Meta-analysis of large studies done on the effects of tight vs. conventional, or more relaxed, glycemic control in type 2 diabetics have failed to demonstrate a difference in all-cause cardiovascular death, non-fatal stroke, or limb amputation, but decreased the risk of nonfatal heart attack by 15%. Additionally, tight glucose control decreased the risk of progression of retinopathy and nephropathy, and decreased the incidence peripheral neuropathy, but increased the risk of hypoglycemia 2.4 times.[22]
Monitoring

Relying on their own perceptions of symptoms of hyperglycemia or hypoglycemia is usually unsatisfactory as mild to moderate hyperglycemia causes no obvious symptoms in nearly all patients. Other considerations include the fact that, while food takes several hours to be digested and absorbed, insulin administration can have glucose lowering effects for as little as 2 hours or 24 hours or more (depending on the nature of the insulin preparation used and individual patient reaction). In addition, the onset and duration of the effects of oral hypoglycemic agents vary from type to type and from patient to patient.
Personal (home) glucose monitoring
Control and outcomes of both types 1 and 2 diabetes may be improved by patients using home glucose meters to regularly measure their glucose levels.[23] Glucose monitoring is both expensive (largely due to the cost of the consumable test strips) and requires significant commitment on the part of the patient. Lifestyle adjustments are generally made by the patients themselves following training by a clinician.
Regular blood testing, especially in type 1 diabetics, is helpful to keep adequate control of glucose levels and to reduce the chance of long term side effects of the disease. There are many (at least 20+) different types of blood monitoring devices available on the market today; not every meter suits all patients and it is a specific matter of choice for the patient, in consultation with a physician or other experienced professional, to find a meter that they personally find comfortable to use. The principle of the devices is virtually the same: a small blood sample is collected and measured. In one type of meter, the electrochemical, a small blood sample is produced by the patient using a lancet (a sterile pointed needle). The blood droplet is usually collected at the bottom of a test strip, while the other end is inserted in the glucose meter. This test strip contains various chemicals so that when the blood is applied, a small electrical charge is created between two contacts. This charge will vary depending on the glucose levels within the blood. In older glucose meters, the drop of blood is placed on top of a strip. A chemical reaction occurs and the strip changes color. The meter then measures the color of the strip optically.
Self-testing is clearly important in type I diabetes where the use of insulin therapy risks episodes of hypoglycemia and home-testing allows for adjustment of dosage on each administration.[24] Its benefit in type 2 diabetes has been more controversial, but recent studies[25] have resulted in guidance[26] that self-monitoring does not improve blood glucose or quality of life.
Benefits of control and reduced hospital admission have been reported.[27] However, patients on oral medication who do not self-adjust their drug dosage will miss many of the benefits of self-testing, and so it is questionable in this group. This is particularly so for patients taking monotherapy with metformin who are not at risk of hypoglycaemia. Regular 6 monthly laboratory testing of HbA1c (glycated haemoglobin) provides some assurance of long-term effective control and allows the adjustment of the patient's routine medication dosages in such cases. High frequency of self-testing in type 2 diabetes has not been shown to be associated with improved control.[28] The argument is made, though, that type 2 patients with poor long term control despite home blood glucose monitoring, either have not had this integrated into their overall management, or are long overdue for tighter control by a switch from oral medication to injected insulin.[29]
Continuous Glucose Monitoring (CGM) CGM technology has been rapidly developing to give people living with diabetes an idea about the speed and direction of their glucose changes. While it still requires calibration from SMBG and is not indicated for use in correction boluses, the accuracy of these monitors is increasing with every innovation. The Libre Blood Sugar Diet Program utilizes the CGM and Libre Sensor and by collecting all the data through a smartphone and smartwatch experts analyze this data 24/7 in Real Time. The results are that certain foods can be identified as causing one's blood sugar levels to rise and other foods as safe foods- that do not make a person's blood sugar levels to rise. Each individual absorbs sugar differently and this is why testing is a necessity.
HbA1c test
A useful test that has usually been done in a laboratory is the measurement of blood HbA1c levels. This is the ratio of glycated hemoglobin in relation to the total hemoglobin. Persistent raised plasma glucose levels cause the proportion of these molecules to go up. This is a test that measures the average amount of diabetic control over a period originally thought to be about 3 months (the average red blood cell lifetime), but more recently thought to be more strongly weighted to the most recent 2 to 4 weeks. In the non-diabetic, the HbA1c level ranges from 4.0 to 6.0%; patients with diabetes mellitus who manage to keep their HbA1c level below 6.5% are considered to have good glycemic control. The HbA1c test is not appropriate if there has been changes to diet or treatment within shorter time periods than 6 weeks or there is disturbance of red cell aging (e.g. recent bleeding or hemolytic anemia) or a hemoglobinopathy (e.g. sickle cell disease). In such cases, the alternative Fructosamine test is used to indicate average control in the preceding 2 to 3 weeks.
Continuous glucose monitoring
The first CGM device made available to consumers was the GlucoWatch biographer in 1999.[30] This product is no longer sold. It was a retrospective device rather than live. Several live monitoring devices have subsequently been manufactured which provide ongoing monitoring of glucose levels on an automated basis during the day.
Electronic health records
Sharing their electronic health records with people who have type 2 diabetes helps them to reduce their blood sugar levels. It is a way of helping people understand their own health condition and involving them actively in its management.[31][32]
m-health monitoring applications
The widespread use of smartphones has turned mobile applications (apps) into a popular means of the usage of all forms of software.[33] As a consequence, the use of mobile apps in managing people's health and supporting their chronic conditions is receiving popularity, especially among healthcare systems, which are showing a great tendency toward using these apps to integrate patient-generated data into electronic health records, and to modify and improve treatment plans accordingly.[34] The number of health-related apps accessible in the App Store and Google Play is approximately 100,000, and among these apps, the ones related to diabetes are the highest in number. Conducting regular self-management tasks such as medication and insulin intake, blood sugar checkup, diet observance, and physical exercise are really demanding.[35] This is why the use of diabetes-related apps for the purposes of recording diet and medication intake or blood glucose level is promising to improve the health condition for the patients. However, despite the high number of apps, the rate of their usage among the patients is not high. One of the reasons for this could be due to the design problems that affect their usability.[36] In addition, a 2016 study of 65 diabetes apps for Android revealed that sensitive data, such as insulin and blood glucose levels, "was routinely collected and shared with third parties".[37][38]
Foot checking
Monitoring a person's feet can help in predicting the likelihood of developing diabetic foot ulcers. A common method for this is using a special thermometer to look for spots on the foot that have higher temperature which indicate the possibility of an ulcer developing.[39] At the same time there is no strong scientific evidence supporting the effectiveness of at-home foot temperature monitoring.[40]
The current guideline in the United Kingdom recommends collecting 8-10 pieces of information for predicting the development of foot ulcers.[41] A simpler method proposed by researchers provides a more detailed risk score based on three pieces of information (insensitivity, foot pulse, previous history of ulcers or amputation). This method is not meant to replace people regularly checking their own feet but complement it.[39][42]
Lifestyle modification
The British National Health Service launched a programme targeting 100,000 people at risk of diabetes to lose weight and take more exercise in 2016. In 2019 it was announced that the programme was successful. The 17,000 people who attended most of the healthy living sessions had, collectively lost nearly 60,000 kg, and the programme was to be doubled in size.[43]
Diet
Because high blood sugar caused by poorly controlled diabetes can lead to a plethora of immediate and long-term complications, it is critical to maintain blood sugars as close to normal as possible, and a diet that produces more controllable glycemic variability is an important factor in producing normal blood sugars.
People with type 1 diabetes who use insulin can eat whatever they want, preferably a healthy diet with some carbohydrate content; in the long term it is helpful to eat a consistent amount of carbohydrate to make blood sugar management easier.[44]
There is a lack of evidence of the usefulness of low-carbohydrate dieting for people with type 1 diabetes.[45] Although for certain individuals it may be feasible to follow a low-carbohydrate regime combined with carefully managed insulin dosing, this is hard to maintain and there are concerns about potential adverse health effects caused by the diet.[45] In general people with type 1 diabetes are advised to follow an individualized eating plan rather than a pre-decided one.[45]
Computer assisted dietary history taking appears to just as applicable as oral or written dietary history taking, however there is lack of evidence showing effects on improving dietary habits, levels of HbA1c and overall management of diabetes.[46]
Exercise
Those who have type two diabetes are prone to having higher than normal blood sugar levels; one way to help manage these levels is through exercise. People diagnosed with type two diabetes can use exercise as a way to maintain their blood sugar and it has been shown to work just as well as medications. Any physical activity can improve type two diabetes, whether that is walking, swimming, or dancing, any type of movement that burns calories.[47]
People living with type two diabetes go through many challenges, one of those challenges is keeping on top of blood glucose levels. Exercise will not only improve blood sugar levels, but can also allow the body to be more sensitive to insulin, reduce the risk of heart disease and stroke which are common illnesses associated with diabetes.[48] By exercising, the body becomes more sensitive to insulin allowing for better absorption of glucose by the muscle cells, not only during but up to 24 hours later as well.[49] Through many studies, it has been made clear that exercise helps with glycemic control and has shown to lower HbA1c levels by approximately 4.2 mmol/mol (0.6%). Studies show that exercise along with diet can slow the rate of impaired glucose tolerance in those with type two diabetes. With that, it is recommended people with type two diabetes take part in 150 minutes on average of exercise a week.[50]
There have not been studies that show how exercise can help manage blood glucose levels in those with type one diabetes. Studies on youth and young adults with type one diabetes where the HBA1c was monitored in both a controlled group and intervention group over a 1-3 month and even up to 5 month program showed no consistent effect on glycemic control. Possible factors that may affect the impact of exercise on management of glucose levels in type one diabetes are that energy consumption increases near time of exercise to account for possible hypoglycaemic episodes; this may be the reason type one diabetics do not see the lowering of glucose levels during exercise.[50] Those with type one diabetes are also prone to nocturnal hypoglycaemic episodes due to exercise as the translocation and expression of GLUT4, which is an insulin-regulated glucose transporter that is used to give glucose to muscle and fat cells, are increased by exercise.[51][52] Those with type one diabetes may be more apprehensive of exercising out of fear of hypoglycaemia.[53] Although exercise may not offer any direct benefit to lower blood glucose levels in those with type one diabetes, there are still many benefits such as a decreased risk of cardiovascular diseases, including blood pressure, lipid profiles, endothelial function, body composition and insulin sensitivity.[53]
The two most effective forms of exercise for people with type two diabetes are aerobic and resistance training.[54] Aerobic exercise has been shown to largely improve HbA1c, and contributes to weight loss and the enhanced metabolic regulation of lipids and lipoproteins.[55] This may be any form of continuous exercise that elevates breathing and heart rate.
During the last 2 decades, resistance training has gained considerable recognition as an optimal form of exercise for patients with type two diabetes.[55] The goal is to build muscle strength by lifting weights, training in calisthenics, yoga, or using weight machines. This form of exercise was linked to a 10% to 15% increase in strength, blood pressure, BMD health, insulin sensitivity, and muscle mass.[55] Current diabetes guidelines recommend strength training two to three times per week in addition to aerobic activities.[56]
The combination of aerobic and resistance training, as recommended by current ADA guidelines, is the most effective when it comes to controlling glucose and lipids in type two diabetes.[56] During a review on 915 adults with diabetes it was reported that combination training was the most effective in reducing HbA1c instead of a singular form of exercise on its own.[57] The American Diabetes Association recommends 150 minutes of moderate to vigorous aerobic exercise a week spread over three to seven days with no more than 2 days between each session paired with 2 to 3 nonconsecutive sessions of strength training. To maximize insulin sensitivity it is recommended to exercise daily. The Association claims that 75 minutes a week is sufficient for most physically fit or younger patients.[56]
Not only does exercising regularly help manage blood sugar levels and weight, it helps reduce the risk of heart attack and stroke, improves cholesterol, reduces risk of diabetes related complications, increases the effect of insulin, provides a boost in energy levels, helps reduce stress and contributes to positive self-esteem.[58] Although incredibly beneficial, the results begin to fade within 48 to 96 hours.Therefore, an ongoing exercise program is required to maintain the health benefits associated with these forms of training.[55]
Medications
Currently, one goal for diabetics is to avoid or minimize chronic diabetic complications, as well as to avoid acute problems of hyperglycemia or hypoglycemia. Adequate control of diabetes leads to lower risk of complications associated with unmonitored diabetes including kidney failure (requiring dialysis or transplant), blindness, heart disease and limb amputation. The most prevalent form of medication is hypoglycemic treatment through either oral hypoglycemics and/or insulin therapy. There is emerging evidence that full-blown diabetes mellitus type 2 can be evaded in those with only mildly impaired glucose tolerance.[59]
Patients with type 1 diabetes mellitus require direct injection of insulin as their bodies cannot produce enough (or even any) insulin. As of 2010, there is no other clinically available form of insulin administration other than injection for patients with type 1: injection can be done by insulin pump, by jet injector, or any of several forms of hypodermic needle. Non-injective methods of insulin administration have been unattainable as the insulin protein breaks down in the digestive tract. There are several insulin application mechanisms under experimental development as of 2004, including a capsule that passes to the liver and delivers insulin into the bloodstream.[60] There have also been proposed vaccines for type I using glutamic acid decarboxylase (GAD), but these are currently not being tested by the pharmaceutical companies that have sublicensed the patents to them.
For type 2 diabetics, diabetic management consists of a combination of diet, exercise, and weight loss, in any achievable combination depending on the patient. Obesity is very common in type 2 diabetes and contributes greatly to insulin resistance. Weight reduction and exercise improve tissue sensitivity to insulin and allow its proper use by target tissues.[61] Patients who have poor diabetic control after lifestyle modifications are typically placed on oral hypoglycemics. Some Type 2 diabetics eventually fail to respond to these and must proceed to insulin therapy. A study conducted in 2008 found that increasingly complex and costly diabetes treatments are being applied to an increasing population with type 2 diabetes. Data from 1994 to 2007 was analyzed and it was found that the mean number of diabetes medications per treated patient increased from 1.14 in 1994 to 1.63 in 2007.[62]
Patient education[63] and compliance with treatment is very important in managing the disease. Improper use of medications and insulin can be very dangerous causing hypo- or hyper-glycemic episodes.
Insulin

For type 1 diabetics, there will always be a need for insulin injections throughout their life, as the pancreatic beta cells of a type 1 diabetic are not capable of producing sufficient insulin. However, both type 1 and type 2 diabetics can see dramatic improvements in blood sugars through modifying their diet, and some type 2 diabetics can fully control the disease by dietary modification.
Insulin therapy requires close monitoring and a great deal of patient education, as improper administration is quite dangerous. For example, when food intake is reduced, less insulin is required. A previously satisfactory dosing may be too much if less food is consumed causing a hypoglycemic reaction if not intelligently adjusted. Exercise decreases insulin requirements as exercise increases glucose uptake by body cells whose glucose uptake is controlled by insulin, and vice versa. In addition, there are several types of insulin with varying times of onset and duration of action.
Several companies are currently working to develop a non-invasive version of insulin, so that injections can be avoided. Mannkind has developed an inhalable version, while companies like Novo Nordisk, Oramed and BioLingus have efforts undergoing for an oral product. Also oral combination products of insulin and a GLP-1 agonist are being developed.
Insulin therapy creates risk because of the inability to continuously know a person's blood glucose level and adjust insulin infusion appropriately. New advances in technology have overcome much of this problem. Small, portable insulin infusion pumps are available from several manufacturers. They allow a continuous infusion of small amounts of insulin to be delivered through the skin around the clock, plus the ability to give bolus doses when a person eats or has elevated blood glucose levels. This is very similar to how the pancreas works, but these pumps lack a continuous "feed-back" mechanism. Thus, the user is still at risk of giving too much or too little insulin unless blood glucose measurements are made.
A further danger of insulin treatment is that while diabetic microangiopathy is usually explained as the result of hyperglycemia, studies in rats indicate that the higher than normal level of insulin diabetics inject to control their hyperglycemia may itself promote small blood vessel disease.[14] While there is no clear evidence that controlling hyperglycemia reduces diabetic macrovascular and cardiovascular disease, there are indications that intensive efforts to normalize blood glucose levels may worsen cardiovascular and cause diabetic mortality.[64]
Driving

Studies conducted in the United States[65] and Europe[66] showed that drivers with type 1 diabetes had twice as many collisions as their non-diabetic spouses, demonstrating the increased risk of driving collisions in the type 1 diabetes population. Diabetes can compromise driving safety in several ways. First, long-term complications of diabetes can interfere with the safe operation of a vehicle. For example, diabetic retinopathy (loss of peripheral vision or visual acuity), or peripheral neuropathy (loss of feeling in the feet) can impair a driver's ability to read street signs, control the speed of the vehicle, apply appropriate pressure to the brakes, etc.
Second, hypoglycemia can affect a person's thinking process, coordination, and state of consciousness.[67][68] This disruption in brain functioning is called neuroglycopenia. Studies have demonstrated that the effects of neuroglycopenia impair driving ability.[67][69] A study involving people with type 1 diabetes found that individuals reporting two or more hypoglycemia-related driving mishaps differ physiologically and behaviorally from their counterparts who report no such mishaps.[70] For example, during hypoglycemia, drivers who had two or more mishaps reported fewer warning symptoms, their driving was more impaired, and their body released less epinephrine (a hormone that helps raise BG). Additionally, individuals with a history of hypoglycemia-related driving mishaps appear to use sugar at a faster rate[71] and are relatively slower at processing information.[72] These findings indicate that although anyone with type 1 diabetes may be at some risk of experiencing disruptive hypoglycemia while driving, there is a subgroup of type 1 drivers who are more vulnerable to such events.
Given the above research findings, it is recommended that drivers with type 1 diabetes with a history of driving mishaps should never drive when their BG is less than 70 mg/dL (3.9 mmol/L). Instead, these drivers are advised to treat hypoglycemia and delay driving until their BG is above 90 mg/dL (5 mmol/L).[70] Such drivers should also learn as much as possible about what causes their hypoglycemia, and use this information to avoid future hypoglycemia while driving.
Studies funded by the National Institutes of Health (NIH) have demonstrated that face-to-face training programs designed to help individuals with type 1 diabetes better anticipate, detect, and prevent extreme BG can reduce the occurrence of future hypoglycemia-related driving mishaps.[73][74][75] An internet-version of this training has also been shown to have significant beneficial results.[76] Additional NIH funded research to develop internet interventions specifically to help improve driving safety in drivers with type 1 diabetes is currently underway.[77]
Exenatide
The U.S. Food and Drug Administration (FDA) has approved a treatment called Exenatide, based on the saliva of a Gila monster, to control blood sugar in patients with type 2 diabetes.
Other regimens
Artificial Intelligence researcher Dr. Cynthia Marling, of the Ohio University Russ College of Engineering and Technology, in collaboration with the Appalachian Rural Health Institute Diabetes Center, is developing a case-based reasoning system to aid in diabetes management. The goal of the project is to provide automated intelligent decision support to diabetes patients and their professional care providers by interpreting the ever-increasing quantities of data provided by current diabetes management technology and translating it into better care without time-consuming manual effort on the part of an endocrinologist or diabetologist.[78] This type of Artificial Intelligence-based treatment shows some promise with initial testing of a prototype system producing best practice treatment advice which anaylizing physicians deemed to have some degree of benefit over 70% of the time and advice of neutral benefit another nearly 25% of the time.[79]
Use of a "Diabetes Coach" is becoming an increasingly popular way to manage diabetes. A Diabetes Coach is usually a Certified diabetes educator (CDE) who is trained to help people in all aspects of caring for their diabetes. The CDE can advise the patient on diet, medications, proper use of insulin injections and pumps, exercise, and other ways to manage diabetes while living a healthy and active lifestyle. CDEs can be found locally or by contacting a company which provides personalized diabetes care using CDEs. Diabetes Coaches can speak to a patient on a pay-per-call basis or via a monthly plan.
Dental care
High blood glucose in diabetic people is a risk factor for developing gum and tooth problems, especially in post-puberty and aging individuals. Diabetic patients have greater chances of developing oral health problems such as tooth decay, salivary gland dysfunction, fungal infections, inflammatory skin disease, periodontal disease or taste impairment and thrush of the mouth.[80] The oral problems in persons with diabetes can be prevented with a good control of the blood sugar levels, regular check-ups and a very good oral hygiene. By maintaining a good oral status, diabetic persons prevent losing their teeth as a result of various periodontal conditions.
Diabetic persons must increase their awareness about oral infections as they have a double impact on health. Firstly, people with diabetes are more likely to develop periodontal disease, which causes increased blood sugar levels, often leading to diabetes complications. Severe periodontal disease can increase blood sugar, contributing to increased periods of time when the body functions with a high blood sugar. This puts diabetics at increased risk for diabetic complications.[81]
The first symptoms of gum and tooth infection in diabetic persons are decreased salivary flow and burning mouth or tongue. Also, patients may experience signs like dry mouth, which increases the incidence of decay. Poorly controlled diabetes usually leads to gum recession, since plaque creates more harmful proteins in the gums.
Tooth decay and cavities are some of the first oral problems that individuals with diabetes are at risk for. Increased blood sugar levels translate into greater sugars and acids that attack the teeth and lead to gum diseases. Gingivitis can also occur as a result of increased blood sugar levels along with an inappropriate oral hygiene. Periodontitis is an oral disease caused by untreated gingivitis and which destroys the soft tissue and bone that support the teeth. This disease may cause the gums to pull away from the teeth which may eventually loosen and fall out. Diabetic people tend to experience more severe periodontitis because diabetes lowers the ability to resist infection[82] and also slows healing. At the same time, an oral infection such as periodontitis can make diabetes more difficult to control because it causes the blood sugar levels to rise.[83]
To prevent further diabetic complications as well as serious oral problems, diabetic persons must keep their blood sugar levels under control and have a proper oral hygiene. A study in the Journal of Periodontology found that poorly controlled type 2 diabetic patients are more likely to develop periodontal disease than well-controlled diabetics are.[81] At the same time, diabetic patients are recommended to have regular checkups with a dental care provider at least once in three to four months. Diabetics who receive good dental care and have good insulin control typically have a better chance at avoiding gum disease to help prevent tooth loss.[84]
Dental care is therefore even more important for diabetic patients than for healthy individuals. Maintaining the teeth and gum healthy is done by taking some preventing measures such as regular appointments at a dentist and a very good oral hygiene. Also, oral health problems can be avoided by closely monitoring the blood sugar levels. Patients who keep better under control their blood sugar levels and diabetes are less likely to develop oral health problems when compared to diabetic patients who control their disease moderately or poorly.
Poor oral hygiene is a great factor to take under consideration when it comes to oral problems and even more in people with diabetes. Diabetic people are advised to brush their teeth at least twice a day, and if possible, after all meals and snacks. However, brushing in the morning and at night is mandatory as well as flossing and using an anti-bacterial mouthwash. Individuals with diabetes are recommended to use toothpaste that contains fluoride as this has proved to be the most efficient in fighting oral infections and tooth decay. Flossing must be done at least once a day, as well because it is helpful in preventing oral problems by removing the plaque between the teeth, which is not removed when brushing.
Diabetic patients must get professional dental cleanings every six months. In cases when dental surgery is needed, it is necessary to take some special precautions such as adjusting diabetes medication or taking antibiotics to prevent infection. Looking for early signs of gum disease (redness, swelling, bleeding gums) and informing the dentist about them is also helpful in preventing further complications. Quitting smoking is recommended to avoid serious diabetes complications and oral diseases.
Diabetic persons are advised to make morning appointments to the dental care provider as during this time of the day the blood sugar levels tend to be better kept under control. Not least, individuals with diabetes must make sure both their physician and dental care provider are informed and aware of their condition, medical history and periodontal status.
Medication nonadherence
Because many patients with diabetes have two or more comorbidities, they often require multiple medications. The prevalence of medication nonadherence is high among patients with chronic conditions, such as diabetes, and nonadherence is associated with public health issues and higher health care costs. One reason for nonadherence is the cost of medications. Being able to detect cost-related nonadherence is important for health care professionals, because this can lead to strategies to assist patients with problems paying for their medications. Some of these strategies are use of generic drugs or therapeutic alternatives, substituting a prescription drug with an over-the-counter medication, and pill-splitting. Interventions to improve adherence can achieve reductions in diabetes morbidity and mortality, as well as significant cost savings to the health care system.[85] Smartphone apps have been found to improve self-management and health outcomes in people with diabetes through functions such as specific reminder alarms,[86] while working with mental health professionals has also been found to help people with diabetes develop the skills to manage their medications and challenges of self-management effectively.[87]
Psychological mechanisms and adherence
As self-management of diabetes typically involves lifestyle modifications, adherence may pose a significant self-management burden on many individuals.[88] For example, individuals with diabetes may find themselves faced with the need to self-monitor their blood glucose levels, adhere to healthier diets and maintain exercise regimens regularly in order to maintain metabolic control and reduce the risk of developing cardiovascular problems. Barriers to adherence have been associated with key psychological mechanisms: knowledge of self-management, beliefs about the efficacy of treatment and self-efficacy/perceived control.[88] Such mechanisms are inter-related, as one's thoughts (e.g. one's perception of diabetes, or one's appraisal of how helpful self-management is) is likely to relate to one's emotions (e.g. motivation to change), which in turn, affects one's self-efficacy (one's confidence in their ability to engage in a behaviour to achieve a desired outcome).[89]
As diabetes management is affected by an individual's emotional and cognitive state, there has been evidence suggesting the self-management of diabetes is negatively affected by diabetes-related distress and depression.[90] There is growing evidence that there is higher levels of clinical depression in patients with diabetes compared to the non-diabetic population.[91][92] Depression in individuals with diabetes has been found to be associated with poorer self-management of symptoms.[93] This suggests that it may be important to target mood in treatment. In the case of children and young people, especially if they are socially disadvantaged, research suggests that it is important that healthcare providers listen to and discuss their feelings and life situation to help them engage with diabetes services and self-management.[94][95]
To this end, treatment programs such as the Cognitive Behavioural Therapy - Adherence and Depression program (CBT-AD)[87] have been developed to target the psychological mechanisms underpinning adherence. By working on increasing motivation and challenging maladaptive illness perceptions, programs such as CBT-AD aim to enhance self-efficacy and improve diabetes-related distress and one's overall quality of life.[96]
Bariatric surgery
While weight loss is clearly beneficial in improving glycemic control in patients with diabetes type 2,[97] maintaining significant weight loss can be a very difficult thing to do. In diabetic people who have a body mass index of 35 or higher, and who have been unable to lose weight otherwise, bariatric surgery offers a viable option to help achieve that goal. In 2018 a Patient-Centered Outcomes Research Institute funded study was published which analyzed the effects of three common types of bariatric surgery on sustained weight loss and long-lasting glycemic control in patients with diabetes type 2.[98] The results of this study demonstrated that, five years after bariatric surgery, there was meaningfully significant weight loss in a large majority of patients. In addition, and more importantly, this study showed that, in type 2 diabetic patients with a body mass index of 35 or higher, bariatric surgery has the potential to lead to complete remission of diabetes in as many as 40% of those people who have the procedure.[99] Like any operation, bariatric surgery is not without risks and complications, and those risks need to weighed against the potential benefits in anyone considering going through with such a procedure.
Research
Type 1 diabetes
Diabetes type 1 is caused by the destruction of enough beta cells to produce symptoms; these cells, which are found in the Islets of Langerhans in the pancreas, produce and secrete insulin, the single hormone responsible for allowing glucose to enter from the blood into cells (in addition to the hormone amylin, another hormone required for glucose homeostasis). Hence, the phrase "curing diabetes type 1" means "causing a maintenance or restoration of the endogenous ability of the body to produce insulin in response to the level of blood glucose" and cooperative operation with counterregulatory hormones.
This section deals only with approaches for curing the underlying condition of diabetes type 1, by enabling the body to endogenously, in vivo, produce insulin in response to the level of blood glucose. It does not cover other approaches, such as, for instance, closed-loop integrated glucometer/insulin pump products, which could potentially increase the quality-of-life for some who have diabetes type 1, and may by some be termed "artificial pancreas".
Encapsulation approach
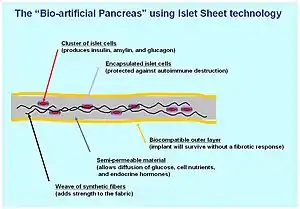
A biological approach to the artificial pancreas is to implant bioengineered tissue containing islet cells, which would secrete the amounts of insulin, amylin and glucagon needed in response to sensed glucose.
When islet cells have been transplanted via the Edmonton protocol, insulin production (and glycemic control) was restored, but at the expense of continued immunosuppression drugs. Encapsulation of the islet cells in a protective coating has been developed to block the immune response to transplanted cells, which relieves the burden of immunosuppression and benefits the longevity of the transplant.[100]
Stem cells
Research is being done at several locations in which islet cells are developed from stem cells.
Stem cell research has also been suggested as a potential avenue for a cure since it may permit regrowth of Islet cells which are genetically part of the treated individual, thus perhaps eliminating the need for immuno-suppressants.[48] This new method autologous nonmyeloablative hematopoietic stem cell transplantation was developed by a research team composed by Brazilian and American scientists (Dr. Julio Voltarelli, Dr. Carlos Eduardo Couri, Dr Richard Burt, and colleagues) and it was the first study to use stem cell therapy in human diabetes mellitus This was initially tested in mice and in 2007 there was the first publication of stem cell therapy to treat this form of diabetes.[101] Until 2009, there was 23 patients included and followed for a mean period of 29.8 months (ranging from 7 to 58 months). In the trial, severe immunosuppression with high doses of cyclophosphamide and anti-thymocyte globulin is used with the aim of "turning off" the immunologic system", and then autologous hematopoietic stem cells are reinfused to regenerate a new one. In summary it is a kind of "immunologic reset" that blocks the autoimmune attack against residual pancreatic insulin-producing cells. Until December 2009, 12 patients remained continuously insulin-free for periods ranging from 14 to 52 months and 8 patients became transiently insulin-free for periods ranging from 6 to 47 months. Of these last 8 patients, 2 became insulin-free again after the use of sitagliptin, a DPP-4 inhibitor approved only to treat type 2 diabetic patients and this is also the first study to document the use and complete insulin-independendce in humans with type 1 diabetes with this medication. In parallel with insulin suspension, indirect measures of endogenous insulin secretion revealed that it significantly increased in the whole group of patients, regardless the need of daily exogenous insulin use.[102]
Gene therapy

Technology for gene therapy is advancing rapidly such that there are multiple pathways possible to support endocrine function, with potential to practically cure diabetes.[103]
- Gene therapy can be used to manufacture insulin directly: an oral medication, consisting of viral vectors containing the insulin sequence, is digested and delivers its genes to the upper intestines. Those intestinal cells will then behave like any viral infected cell, and will reproduce the insulin protein. The virus can be controlled to infect only the cells which respond to the presence of glucose, such that insulin is produced only in the presence of high glucose levels. Due to the limited numbers of vectors delivered, very few intestinal cells would actually be impacted and would die off naturally in a few days. Therefore, by varying the amount of oral medication used, the amount of insulin created by gene therapy can be increased or decreased as needed. As the insulin-producing intestinal cells die off, they are boosted by additional oral medications.[104]
- Gene therapy might eventually be used to cure the cause of beta cell destruction, thereby curing the new diabetes patient before the beta cell destruction is complete and irreversible.[105]
- Gene therapy can be used to turn duodenum cells and duodenum adult stem cells into beta cells which produce insulin and amylin naturally. By delivering beta cell DNA to the intestine cells in the duodenum, a few intestine cells will turn into beta cells, and subsequently adult stem cells will develop into beta cells. This makes the supply of beta cells in the duodenum self-replenishing, and the beta cells will produce insulin in proportional response to carbohydrates consumed.[106]
Monoclonal antibodies
In November 2022 the FDA approved Teplizumab a monoclonal antibody drug which aims to delay type 1 diabetes by reprogramming the immune system to stop mistakenly attacking pancreatic cells.[107][108]
Type 2 diabetes
Type 2 diabetes is usually first treated by increasing physical activity, and eliminating saturated fat and reducing sugar and carbohydrate intake with a goal of losing weight. These can restore insulin sensitivity even when the weight loss is modest, for example around 5 kg (10 to 15 lb), most especially when it is in abdominal fat deposits. Diets that are very low in saturated fats have been claimed to reverse insulin resistance.[109][110]
Cognitive Behavioural Therapy is an effective intervention for improving adherence to medication, depression and glycaemic control, with enduring and clinically meaningful benefits for diabetes self-management and glycaemic control in adults with type 2 diabetes and comorbid depression.[96]
Testosterone replacement therapy may improve glucose tolerance and insulin sensitivity in diabetic hypogonadal men. The mechanisms by which testosterone decreases insulin resistance is under study.[111] Moreover, testosterone may have a protective effect on pancreatic beta cells, which is possibly exerted by androgen-receptor-mediated mechanisms and influence of inflammatory cytokines.[112]
Recently it has been suggested that a type of gastric bypass surgery may normalize blood glucose levels in 80–100% of severely obese patients with diabetes. The precise causal mechanisms are being intensively researched; its results may not simply be attributable to weight loss, as the improvement in blood sugars seems to precede any change in body mass. This approach may become a treatment for some people with type 2 diabetes, but has not yet been studied in prospective clinical trials.[113] This surgery may have the additional benefit of reducing the death rate from all causes by up to 40% in severely obese people.[114] A small number of normal to moderately obese patients with type 2 diabetes have successfully undergone similar operations.[115][116]
MODY is a rare genetic form of diabetes, often mistaken for Type 1 or Type 2. The medical management is variable and depends on each individual case.[117]
Several immunosuppressive drugs targeting the chronic inflammation in type 2 diabetes have been tested.[118]
See also
References
- Simó R, Hernández C (August 2002). "[Treatment of diabetes mellitus: general goals, and clinical practice management]". Revista Espanola de Cardiologia. 55 (8): 845–860. doi:10.1016/s0300-8932(02)76714-6. PMID 12199981.
- "Practice Guidelines Resources". American Diabetes Association. Retrieved 2023-07-12.
- Hur, Kyu Yeon; Moon, Min Kyong; Park, Jong Suk; Kim, Soo-Kyung; Lee, Seung-Hwan; Yun, Jae-Seung; Baek, Jong Ha; Noh, Junghyun; Lee, Byung-Wan; Oh, Tae Jung; Chon, Suk; Yang, Ye Seul; Son, Jang Won; Choi, Jong Han; Song, Kee Ho; Kim, Nam Hoon; Kim, Sang Yong; Kim, Jin Wha; Rhee, Sang Youl; Lee, You-Bin (2021-07-31). "2021 Clinical Practice Guidelines for Diabetes Mellitus of the Korean Diabetes Association". Diabetes & Metabolism Journal. Korean Diabetes Association. 45 (4): 461–481. doi:10.4093/dmj.2021.0156. ISSN 2233-6079. PMC 8369224. PMID 34352984.
- American Diabetes Association (January 2019). "6. Glycemic Targets: Standards of Medical Care in Diabetes-2019". Diabetes Care. 42 (Suppl 1): S61–S70. doi:10.2337/dc19-S006. PMID 30559232.
- Qaseem A, Vijan S, Snow V, Cross JT, Weiss KB, Owens DK (September 2007). "Glycemic control and type 2 diabetes mellitus: the optimal hemoglobin A1c targets. A guidance statement from the American College of Physicians". Annals of Internal Medicine. 147 (6): 417–422. doi:10.7326/0003-4819-147-6-200709180-00012. PMID 17876024.
- Brown AF, Mangione CM, Saliba D, Sarkisian CA (May 2003). "Guidelines for improving the care of the older person with diabetes mellitus". Journal of the American Geriatrics Society. 51 (5 Suppl Guidelines): S265–S280. doi:10.1046/j.1532-5415.51.5s.1.x. PMID 12694461. S2CID 9149226.
- Arora KS, Binjoo N, Reddy GV, Kaur P, Modgil R, Negi LS (2015-01-01). "Determination of normal range for fasting salivary glucose in Type 1 diabetics". Journal of International Society of Preventive & Community Dentistry. 5 (5): 377–382. doi:10.4103/2231-0762.165923. PMC 4606601. PMID 26539389.
- Briscoe VJ, Davis SN (2006). "Hypoglycemia in Type 1 and Type 2 Diabetes: Physiology, Pathophysiology, and Management". Clinical Diabetes. 24 (3): 115–21. doi:10.2337/diaclin.24.3.115.
- Fishbein H, Palumbo P (1995). "Acute Metabolic Complications in Diabetes". Diabetes in America. Bethesda: National Diabetes Data Group. p. 283.
- Asuncion MM, Shaheen M, Ganesan K, Velasques J, Teklehaimanot S, Pan D, Norris K (2007). "Increase in hypoglycemic admissions: California hospital discharge data". Ethnicity & Disease. 17 (3): 536–540. PMID 17985510.
- Perlmuter LC, Flanagan BP, Shah PH, Singh SP (October 2008). "Glycemic control and hypoglycemia: is the loser the winner?". Diabetes Care. 31 (10): 2072–2076. doi:10.2337/dc08-1441. PMC 2551657. PMID 18820231.
- Tupola S, Rajantie J, Mäenpää J (August 1998). "Severe hypoglycaemia in children and adolescents during multiple-dose insulin therapy". Diabetic Medicine. 15 (8): 695–699. doi:10.1002/(SICI)1096-9136(199808)15:8<695::AID-DIA651>3.0.CO;2-C. PMID 9702475. S2CID 38883129.
- Fujioka M, Okuchi K, Hiramatsu KI, Sakaki T, Sakaguchi S, Ishii Y (March 1997). "Specific changes in human brain after hypoglycemic injury". Stroke. 28 (3): 584–587. doi:10.1161/01.STR.28.3.584. PMID 9056615.
- Sugimoto K, Baba M, Suda T, Yasujima M, Yagihashi S (2003). "Peripheral neuropathy and microangiopathy in rats with insulinoma: association with chronic hyperinsulinemia". Diabetes/Metabolism Research and Reviews. 19 (5): 392–400. doi:10.1002/dmrr.395. PMID 12951647. S2CID 41619772.
- Tarnow L, Groop PH, Hadjadj S, Kazeem G, Cambien F, Marre M, et al. (January 2008). "European rational approach for the genetics of diabetic complications--EURAGEDIC: patient populations and strategy". Nephrology, Dialysis, Transplantation. 23 (1): 161–168. doi:10.1093/ndt/gfm501. PMID 17704113.
- Murdolo, G.; Kempf, K.; Hammarstedt, A.; Herder, C.; Smith, U.; Jansson, P. A. (2007-09-01). "Insulin differentially modulates the peripheral endocannabinoid system in human subcutaneous abdominal adipose tissue from lean and obese individuals". Journal of Endocrinological Investigation. 30 (8): RC17–RC21. doi:10.1007/BF03347440. ISSN 1720-8386. PMID 17923791. S2CID 39337082.
- Adams DD (June 2008). "Autoimmune destruction of pericytes as the cause of diabetic retinopathy". Clinical Ophthalmology. 2 (2): 295–298. doi:10.2147/OPTH.S2629. PMC 2693966. PMID 19668719.
- Huang ES, Brown SE, Ewigman BG, Foley EC, Meltzer DO (October 2007). "Patient perceptions of quality of life with diabetes-related complications and treatments". Diabetes Care. 30 (10): 2478–2483. doi:10.2337/dc07-0499. PMC 2288662. PMID 17623824.
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. (March 2015). "Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes". Diabetologia. 58 (3): 429–442. doi:10.1007/s00125-014-3460-0. PMID 25583541.
- "Standards of medical care in diabetes--2015: summary of revisions". Diabetes Care. 38 (38): S4. January 2015. doi:10.2337/dc15-S003. PMID 25537706.
- Makam AN, Nguyen OK (January 2017). "An Evidence-Based Medicine Approach to Antihyperglycemic Therapy in Diabetes Mellitus to Overcome Overtreatment". Circulation. 135 (2): 180–195. doi:10.1161/CIRCULATIONAHA.116.022622. PMC 5502688. PMID 28069712.
- Buehler AM, Cavalcanti AB, Berwanger O, Figueiro M, Laranjeira LN, Zazula AD, et al. (June 2013). "Effect of tight blood glucose control versus conventional control in patients with type 2 diabetes mellitus: a systematic review with meta-analysis of randomized controlled trials". Cardiovascular Therapeutics. 31 (3): 147–160. doi:10.1111/j.1755-5922.2011.00308.x. PMID 22212499.
- "Blood glucose and blood sugar are interchangeable terms, and both are crucial to the health of the body; especially for people with diabetes". Diabetes. 2019-01-15. Retrieved 2021-09-12.
- Evans JM, Newton RW, Ruta DA, MacDonald TM, Stevenson RJ, Morris AD (July 1999). "Frequency of blood glucose monitoring in relation to glycaemic control: observational study with diabetes database". BMJ. 319 (7202): 83–86. doi:10.1136/bmj.319.7202.83. PMC 28155. PMID 10398627.
- Young LA, Buse JB, Weaver MA, Vu MB, Mitchell CM, Blakeney T, et al. (July 2017). "Glucose Self-monitoring in Non-Insulin-Treated Patients With Type 2 Diabetes in Primary Care Settings: A Randomized Trial". JAMA Internal Medicine. 177 (7): 920–929. doi:10.1001/jamainternmed.2017.1233. PMC 5818811. PMID 28600913.
- Perry D, Moe S, Korownyk C, Lindblad AJ, Kolber MR, Thomas B, et al. (April 2019). "Top studies relevant to primary care from 2018: From PEER". Canadian Family Physician. 65 (4): 260–263. PMC 6467664. PMID 30979756.
- Kibriya MG, Ali L, Banik NG, Khan AK (December 1999). "Home monitoring of blood glucose (HMBG) in Type-2 diabetes mellitus in a developing country". Diabetes Research and Clinical Practice. 46 (3): 253–257. doi:10.1016/S0168-8227(99)00093-5. PMID 10624792.
- Jaworska J, Dziemidok P, Kulik TB, Rudnicka-Drozak E (2004). "Frequency of self-monitoring and its effect on metabolic control in patients with type 2 diabetes". Annales Universitatis Mariae Curie-Sklodowska. Sectio D. 59 (1): 310–316. PMID 16146003.
- Roach P (December 2004). "Better systems, not guidelines, for glucose monitoring". BMJ. 329 (7479): E332. doi:10.1136/bmj.329.7479.E332. PMID 15591539. S2CID 36641531.
- "History of Glucose Monitoring" (PDF). American Diabetes Association. Retrieved 6 October 2020.
- Neves AL, Freise L, Laranjo L, Carter AW, Darzi A, Mayer E (December 2020). "Impact of providing patients access to electronic health records on quality and safety of care: a systematic review and meta-analysis". BMJ Quality & Safety. 29 (12): 1019–1032. doi:10.1136/bmjqs-2019-010581. PMC 7785164. PMID 32532814.
- "Sharing electronic records with patients led to improved control of type two diabetes". NIHR Evidence (Plain English summary). 2020-10-21. doi:10.3310/alert_42103. S2CID 242149388.
- Jeong JW, Kim NH, In HP (July 2020). "Detecting usability problems in mobile applications on the basis of dissimilarity in user behavior". International Journal of Human-Computer Studies. 139: 102364. doi:10.1016/j.ijhcs.2019.10.001. S2CID 208105117.
- Sarkar U, Gourley GI, Lyles CR, Tieu L, Clarity C, Newmark L, et al. (December 2016). "Usability of Commercially Available Mobile Applications for Diverse Patients". Journal of General Internal Medicine. 31 (12): 1417–1426. doi:10.1007/s11606-016-3771-6. PMC 5130945. PMID 27418347.
- Hood M, Wilson R, Corsica J, Bradley L, Chirinos D, Vivo A (December 2016). "What do we know about mobile applications for diabetes self-management? A review of reviews". Journal of Behavioral Medicine. 39 (6): 981–994. doi:10.1007/s10865-016-9765-3. PMID 27412774. S2CID 29465893.
- Fu HN, Adam TJ, Konstan JA, Wolfson JA, Clancy TR, Wyman JF (April 2019). "Influence of Patient Characteristics and Psychological Needs on Diabetes Mobile App Usability in Adults With Type 1 or Type 2 Diabetes: Crossover Randomized Trial". JMIR Diabetes. 4 (2): e11462. doi:10.2196/11462. PMC 6660121. PMID 31038468.
- "Health apps may pose major privacy concerns". www.cbsnews.com. 8 March 2016. Retrieved 2020-10-07.
- "Health apps and the sharing of information with third parties". ScienceDaily. Retrieved 2020-10-07.
- "Simple tool identifies the people with diabetes most likely to develop foot ulcers". NIHR Evidence. National Institute for Health and Care Research. 2022-06-21. doi:10.3310/nihrevidence_51316. S2CID 251787297.
- Golledge, Jonathan; Fernando, Malindu E; Alahakoon, Chanika; Lazzarini, Peter A.; aan de Stegge, Wouter B.; van Netten, Jaap J.; Bus, Sicco A. (23 May 2022). "Efficacy of at home monitoring of foot temperature for risk reduction of diabetes‐related foot ulcer: A meta‐analysis". Diabetes/Metabolism Research and Reviews. 38 (6): e3549. doi:10.1002/dmrr.3549. ISSN 1520-7552. PMC 9541448. PMID 35605998. S2CID 251981184.
- "Diabetic foot problems: prevention and management". National Institute for Health and Care Excellence (NICE). 26 August 2015. Retrieved 2022-09-06.
- Chappell, Francesca M; Crawford, Fay; Horne, Margaret; Leese, Graham P; Martin, Angela; Weller, David; Boulton, Andrew J M; Abbott, Caroline; Monteiro-Soares, Matilde; Veves, Aristidis; Riley, Richard D (25 May 2021). "Development and validation of a clinical prediction rule for development of diabetic foot ulceration: an analysis of data from five cohort studies". BMJ Open Diabetes Research & Care. 9 (1): e002150. doi:10.1136/bmjdrc-2021-002150. ISSN 2052-4897. PMC 8154962. PMID 34035053.
- "Government set to double NHS diabetes prevention programme". Pulse. 2 April 2019. Retrieved 13 May 2019.
- "I have Type 1 diabetes - what can I eat?". Diabetes UK. Retrieved 14 June 2019.
- Seckold R, Fisher E, de Bock M, King BR, Smart CE (March 2019). "The ups and downs of low-carbohydrate diets in the management of Type 1 diabetes: a review of clinical outcomes". Diabetic Medicine (Review). 36 (3): 326–334. doi:10.1111/dme.13845. PMID 30362180. S2CID 53102654.
Low‐carbohydrate diets are of interest for improving glycaemic outcomes in the management of Type 1 diabetes. There is limited evidence to support their routine use in the management of Type 1 diabetes.
- Wei I, Pappas Y, Car J, Sheikh A, Majeed A, et al. (Cochrane Metabolic and Endocrine Disorders Group) (December 2011). "Computer-assisted versus oral-and-written dietary history taking for diabetes mellitus". The Cochrane Database of Systematic Reviews. 2011 (12): CD008488. doi:10.1002/14651858.CD008488.pub2. PMC 6486022. PMID 22161430.
- "Physical activity". DiabetesCanadaWebsite. Retrieved 2022-11-20.
- CDC (2022-11-03). "Get Active". Centers for Disease Control and Prevention. Retrieved 2022-11-20.
- "Blood Sugar and Exercise | ADA". diabetes.org. Retrieved 2022-11-20.
- Chimen, M.; Kennedy, A.; Nirantharakumar, K.; Pang, T. T.; Andrews, R.; Narendran, P. (2012-03-01). "What are the health benefits of physical activity in type 1 diabetes mellitus? A literature review". Diabetologia. 55 (3): 542–551. doi:10.1007/s00125-011-2403-2. ISSN 1432-0428. PMID 22189486. S2CID 21040215.
- Abushamat, Layla A.; McClatchey, P. Mason; Scalzo, Rebecca L.; Reusch, Jane E. B. (2000), Feingold, Kenneth R.; Anawalt, Bradley; Boyce, Alison; Chrousos, George (eds.), "The Role of Exercise in Diabetes", Endotext, South Dartmouth (MA): MDText.com, Inc., PMID 31751111, retrieved 2022-11-20
- Stöckli, Jacqueline; Fazakerley, Daniel J.; James, David E. (2011-12-15). "GLUT4 exocytosis". Journal of Cell Science. 124 (24): 4147–4159. doi:10.1242/jcs.097063. ISSN 0021-9533. PMC 3258103. PMID 22247191.
- Lumb, Alistair (2014-12-01). "Diabetes and exercise". Clinical Medicine. 14 (6): 673–676. doi:10.7861/clinmedicine.14-6-673. ISSN 1470-2118. PMC 4954144. PMID 25468857.
- "Exercise & activity". DiabetesCanadaWebsite. Retrieved 2022-12-01.
- KIRWAN, JOHN P.; SACKS, JESSICA; NIEUWOUDT, STEPHAN (July 2017). "The essential role of exercise in the management of type 2 diabetes". Cleveland Clinic Journal of Medicine. 84 (7 Suppl 1): S15–S21. doi:10.3949/ccjm.84.s1.03. ISSN 0891-1150. PMC 5846677. PMID 28708479.
- "My Site - Chapter 10: Physical Activity and Diabetes". guidelines.diabetes.ca. Retrieved 2022-12-01.
- Schwingshackl, Lukas; Missbach, Benjamin; Dias, Sofia; König, Jürgen; Hoffmann, Georg (2014-09-01). "Impact of different training modalities on glycaemic control and blood lipids in patients with type 2 diabetes: a systematic review and network meta-analysis". Diabetologia. 57 (9): 1789–1797. doi:10.1007/s00125-014-3303-z. ISSN 1432-0428. PMID 24996616. S2CID 24422319.
- "Managing Diabetes". DRIF. Retrieved 2022-12-01.
- Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, et al. (May 2001). "Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance". The New England Journal of Medicine. 344 (18): 1343–1350. doi:10.1056/NEJM200105033441801. PMID 11333990.
- Oramed Pharmaceuticals (2014-09-25). "Making insulin delivery in capsule form a reality".
- Mealey BL (2006). "Diabetes Mellitus Management". Diabetes Mellitus and Oral Health. Armenian Medical Network. Retrieved 2 October 2009.
- Alexander GC, Sehgal NL, Moloney RM, Stafford RS (October 2008). "National trends in treatment of type 2 diabetes mellitus, 1994-2007". Archives of Internal Medicine. 168 (19): 2088–2094. doi:10.1001/archinte.168.19.2088. PMC 2868588. PMID 18955637.
- Mannucci E, Giaccari A, Gallo M, Bonifazi A, Belén ÁD, Masini ML, et al. (February 2022). "Self-management in patients with type 2 diabetes: Group-based versus individual education. A systematic review with meta-analysis of randomized trails". Nutrition, Metabolism, and Cardiovascular Diseases. 32 (2): 330–336. doi:10.1016/j.numecd.2021.10.005. PMID 34893413. S2CID 244580173.
- Mudaliar S (September 2009). "Serum glucose control in diabetic patients with cardiovascular disease: should we be less aggressive?". Current Atherosclerosis Reports. 11 (5): 384–390. doi:10.1007/s11883-009-0058-y. PMID 19664383. S2CID 40585617.
- Songer, TJ. Low blood sugar and motor vehicle crashes in persons with type 1 diabetes, Annu Proc Assoc Adv Automotive Med, 46:424–27 (2002)
- Cox DJ, Penberthy JK, Zrebiec J, Weinger K, Aikens JE, Frier B, et al. (August 2003). "Diabetes and driving mishaps: frequency and correlations from a multinational survey". Diabetes Care. 26 (8): 2329–2334. doi:10.2337/diacare.26.8.2329. PMID 12882857.
- Cox DJ, Gonder-Frederick L, Clarke W (February 1993). "Driving decrements in type I diabetes during moderate hypoglycemia". Diabetes. 42 (2): 239–243. doi:10.2337/diabetes.42.2.239. PMID 8425660.
- Clarke WL, Cox DJ, Gonder-Frederick LA, Kovatchev B (August 1999). "Hypoglycemia and the decision to drive a motor vehicle by persons with diabetes". JAMA. 282 (8): 750–754. doi:10.1001/jama.282.8.750. PMID 10463710.
- Cox DJ, Gonder-Frederick LA, Kovatchev BP, Julian DM, Clarke WL (February 2000). "Progressive hypoglycemia's impact on driving simulation performance. Occurrence, awareness and correction". Diabetes Care. 23 (2): 163–170. doi:10.2337/diacare.23.2.163. PMID 10868825.
- Cox DJ, Kovatchev BP, Anderson SM, Clarke WL, Gonder-Frederick LA (November 2010). "Type 1 diabetic drivers with and without a history of recurrent hypoglycemia-related driving mishaps: physiological and performance differences during euglycemia and the induction of hypoglycemia". Diabetes Care. 33 (11): 2430–2435. doi:10.2337/dc09-2130. PMC 2963507. PMID 20699432.
- Cox DJ, Gonder-Frederick LA, Kovatchev BP, Clarke WL (2002). "The metabolic demands of driving for drivers with type 1 diabetes mellitus". Diabetes/Metabolism Research and Reviews. 18 (5): 381–385. doi:10.1002/dmrr.306. PMID 12397580. S2CID 25094659.
- Campbell LK, Gonder-Frederick LA, Broshek DK, Kovatchev BP, Anderson S, Clarke WL, Cox DJ (August 2010). "Neurocognitive Differences Between Drivers with Type 1 Diabetes with and without a Recent History of Recurrent Driving Mishaps". International Journal of Diabetes Mellitus. 2 (2): 73–77. doi:10.1016/j.ijdm.2010.05.014. PMC 2993428. PMID 21127720.
- Cox DJ, Gonder-Frederick L, Julian DM, Clarke W (January 1994). "Long-term follow-up evaluation of blood glucose awareness training". Diabetes Care. 17 (1): 1–5. doi:10.2337/diacare.17.1.1. PMID 8112183. S2CID 44443393.
- Cox DJ, Gonder-Frederick L, Polonsky W, Schlundt D, Kovatchev B, Clarke W (April 2001). "Blood glucose awareness training (BGAT-2): long-term benefits". Diabetes Care. 24 (4): 637–642. doi:10.2337/diacare.24.4.637. PMID 11315822.
- Broers S, le Cessie S, van Vliet KP, Spinhoven P, van der Ven NC, Radder JK (February 2002). "Blood Glucose Awareness Training in Dutch Type 1 diabetes patients. Short-term evaluation of individual and group training". Diabetic Medicine. 19 (2): 157–161. doi:10.1046/j.1464-5491.2002.00682.x. PMID 11874433. S2CID 36654333.
- Cox D, Ritterband L, Magee J, Clarke W, Gonder-Frederick L (August 2008). "Blood glucose awareness training delivered over the internet". Diabetes Care. 31 (8): 1527–1528. doi:10.2337/dc07-1956. PMC 2494647. PMID 18477813.
- http://www.DiabetesDriving.com Diabetes Driving.
- Schwartz FL, Shubrook JH, Marling CR (July 2008). "Use of case-based reasoning to enhance intensive management of patients on insulin pump therapy". Journal of Diabetes Science and Technology. 2 (4): 603–611. doi:10.1177/193229680800200411. PMC 2769779. PMID 19885236.
- Walker D (November 2007). "Similarity Determination and Case Retrieval in an Intelligent Decision Support System for Diabetes Management" (PDF). Retrieved 2 October 2009.
- "Oral diabetes care". Retrieved 2010-05-05.
- "Gum Disease and Diabetes". Archived from the original on 2010-06-12. Retrieved 2010-05-05.
- Koh GC, Peacock SJ, van der Poll T, Wiersinga WJ (April 2012). "The impact of diabetes on the pathogenesis of sepsis". European Journal of Clinical Microbiology & Infectious Diseases. 31 (4): 379–388. doi:10.1007/s10096-011-1337-4. PMC 3303037. PMID 21805196.
- "Diabetes and Dental Care: Guide to a Healthy Mouth". Retrieved 2010-05-05.
- "Diabetes and Oral Health". Archived from the original on 2010-04-24. Retrieved 2010-05-05.
- Chan M (2010). "Reducing cost-related medication nonadherence in patients with diabetes". Drug Benefit Trends. 22: 67–71.
- Cui M, Wu X, Mao J, Wang X, Nie M (2016). "T2DM Self-Management via Smartphone Applications: A Systematic Review and Meta-Analysis". PLOS ONE. 11 (11): e0166718. Bibcode:2016PLoSO..1166718C. doi:10.1371/journal.pone.0166718. PMC 5115794. PMID 27861583.
- Safren SA, Gonzalez JS, Wexler DJ, Psaros C, Delahanty LM, Blashill AJ, et al. (2013). "A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in patients with uncontrolled type 2 diabetes". Diabetes Care. 37 (3): 625–633. doi:10.2337/dc13-0816. PMC 3931377. PMID 24170758.
- Gonzalez JS, Tanenbaum ML, Commissariat PV (October 2016). "Psychosocial factors in medication adherence and diabetes self-management: Implications for research and practice". The American Psychologist. 71 (7): 539–551. doi:10.1037/a0040388. PMC 5792162. PMID 27690483.
- Chew BH, Vos RC, Metzendorf MI, Scholten RJ, Rutten GE (September 2017). Chew BH (ed.). "Psychological interventions for diabetes-related distress in adults with type 2 diabetes mellitus". The Cochrane Database of Systematic Reviews. 9 (9): CD011469. doi:10.1002/14651858.CD011469. PMC 6483710. PMID 28954185.
- Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE (July 2000). "Depression and poor glycemic control: a meta-analytic review of the literature". Diabetes Care. 23 (7): 934–942. doi:10.2337/diacare.23.7.934. PMID 10895843.
- Hussain S, Habib A, Singh A, Akhtar M, Najmi AK (December 2018). "Prevalence of depression among type 2 diabetes mellitus patients in India: A meta-analysis". Psychiatry Research. 270: 264–273. doi:10.1016/j.psychres.2018.09.037. PMID 30273857. S2CID 52919905.
- Ali S, Stone MA, Peters JL, Davies MJ, Khunti K (November 2006). "The prevalence of co-morbid depression in adults with Type 2 diabetes: a systematic review and meta-analysis". Diabetic Medicine. 23 (11): 1165–1173. doi:10.1111/j.1464-5491.2006.01943.x. PMID 17054590. S2CID 25685073.
- Gonzalez JS, Peyrot M, McCarl LA, Collins EM, Serpa L, Mimiaga MJ, Safren SA (December 2008). "Depression and diabetes treatment nonadherence: a meta-analysis". Diabetes Care. 31 (12): 2398–2403. doi:10.2337/dc08-1341. PMC 2584202. PMID 19033420.
- "Why don't children and young people engage with diabetes services?". NIHR Evidence (Plain English summary). National Institute for Health and Care Research. 2022-03-15. doi:10.3310/alert_49448. S2CID 247483863.
- Sharpe D, Rajabi M, Harden A, Moodambail AR, Hakeem V (October 2021). "Supporting disengaged children and young people living with diabetes to self-care: a qualitative study in a socially disadvantaged and ethnically diverse urban area". BMJ Open. 11 (10): e046989. doi:10.1136/bmjopen-2020-046989. PMC 8515452. PMID 34645656.
- Safren SA, Gonzalez JS, Wexler DJ, Psaros C, Delahanty LM, Blashill AJ, et al. (2014). "A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in patients with uncontrolled type 2 diabetes". Diabetes Care. 37 (3): 625–633. doi:10.2337/dc13-0816. PMC 3931377. PMID 24170758.
- McCombie, Louise; Leslie, Wilma; Taylor, Roy; Kennon, Brian; Sattar, Naveed; Lean, Mike E. J. (2017-09-13). "Beating type 2 diabetes into remission". BMJ. 358: j4030. doi:10.1136/bmj.j4030. ISSN 0959-8138. PMID 28903916. S2CID 28182743.
- Arterburn, David; Wellman, Robert; Emiliano, Ana; Smith, Steven R.; Odegaard, Andrew O.; Murali, Sameer; Williams, Neely; Coleman, Karen J.; Courcoulas, Anita; Coley, R. Yates; Anau, Jane; Pardee, Roy; Toh, Sengwee; Janning, Cheri; Cook, Andrea (2018-12-04). "Comparative Effectiveness and Safety of Bariatric Procedures for Weight Loss: A PCORnet Cohort Study". Annals of Internal Medicine. 169 (11): 741–750. doi:10.7326/M17-2786. ISSN 0003-4819. PMC 6652193. PMID 30383139.
- Anau, Jane; Arterburn, David; Coleman, Karen J.; Coley, R. Yates; Cook, Andrea J.; Courcoulas, Anita; Janning, Cheri; McTigue, Kathleen; Pardee, Roy; Toh, Sengwee; Wellman, Robert; Williams, Neely (2020-11-02). "Comparing Three Types of Weight Loss Surgery—The PCORnet Bariatric Study". doi:10.25302/11.2020.obs.150530683. S2CID 228814682.
{{cite journal}}: Cite journal requires|journal=(help) - Cerco Medical: Science: Methods Archived 2009-01-15 at the Wayback Machine
- Voltarelli JC, Couri CE, Stracieri AB, Oliveira MC, Moraes DA, Pieroni F, et al. (April 2007). "Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus". JAMA. 297 (14): 1568–1576. doi:10.1001/jama.297.14.1568. PMID 17426276.
- Couri CE, Oliveira MC, Stracieri AB, Moraes DA, Pieroni F, Barros GM, et al. (April 2009). "C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus". JAMA. 301 (15): 1573–1579. doi:10.1001/jama.2009.470. PMID 19366777.
- Gene Therapy Approaches to Diabetes Archived 2009-10-29 at the Wayback Machine
- Mary Ann Liebert, Inc.
- hopkinsbayview.org
- Engene Inc
- American Diabetes Association Professional Practice Committee (1 January 2022). "2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022". Diabetes Care. 45 (45): S17–S38. doi:10.2337/dc22-S002. PMID 34964875. S2CID 245451959. Retrieved 17 November 2022.
- "Game-changing type 1 diabetes drug approved in US". BBC News. 2022-11-18. Retrieved 2022-11-20.
- Barnard N (2007). "13". Dr. Neal Barnard's Program for Reversing Diabetes: The Scientifically Proven System for Reversing Diabetes Without Drugs. New York, NY: Rodale/Holtzbrinck Publishers. ISBN 978-1-59486-528-2.
- Barnard ND, Katcher HI, Jenkins DJ, Cohen J, Turner-McGrievy G (May 2009). "Vegetarian and vegan diets in type 2 diabetes management". Nutrition Reviews. 67 (5): 255–263. doi:10.1111/j.1753-4887.2009.00198.x. PMID 19386029. S2CID 1662675.
- Traish AM, Saad F, Guay A (2009). "The dark side of testosterone deficiency: II. Type 2 diabetes and insulin resistance". Journal of Andrology. 30 (1): 23–32. doi:10.2164/jandrol.108.005751. PMID 18772488. S2CID 29463129.
- Zitzmann M (December 2009). "Testosterone deficiency, insulin resistance and the metabolic syndrome". Nature Reviews. Endocrinology. 5 (12): 673–681. doi:10.1038/nrendo.2009.212. PMID 19859074. S2CID 22307175.
- Rubino F, Gagner M (November 2002). "Potential of surgery for curing type 2 diabetes mellitus". Annals of Surgery. 236 (5): 554–559. doi:10.1097/00000658-200211000-00003. PMC 1422611. PMID 12409659.
- Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, et al. (August 2007). "Long-term mortality after gastric bypass surgery". The New England Journal of Medicine. 357 (8): 753–761. doi:10.1056/NEJMoa066603. PMID 17715409. S2CID 8710295.
- Cohen RV, Schiavon CA, Pinheiro JS, Correa JL, Rubino F (2007). "Duodenal-jejunal bypass for the treatment of type 2 diabetes in patients with body mass index of 22-34 kg/m2: a report of 2 cases". Surgery for Obesity and Related Diseases. 3 (2): 195–197. doi:10.1016/j.soard.2007.01.009. PMID 17386401.
- Vasonconcelos A (September 2007). "Could type 2 diabetes be reversed using surgery?". New Scientist (2619): 11–13. Retrieved 26 September 2007.
- Elkholy S, Lardhi AA (2015-05-01). "Do we need to test for maturity onset diabetes of the young among newly diagnosed diabetics in Saudi Arabia?". International Journal of Diabetes Mellitus. 3 (1): 51–56. doi:10.1016/j.ijdm.2011.01.006.
- Mikkelsen RR, Hundahl MP, Torp CK, Rodríguez-Carrio J, Kjolby M, Bruun JM, Kragstrup TW (2022). "Immunomodulatory and immunosuppressive therapies in cardiovascular disease and type 2 diabetes mellitus: A bedside-to-bench approach". Eur J Pharmacol. 925: 174998. doi:10.1016/j.ejphar.2022.174998. PMID 35533739. S2CID 248589827.
External links
- American College of Physicians Diabetes Portal – Resources for patients and clinicians
- American Diabetes Association
- Prevent Diabetes Problems: Keep Your Diabetes Under Control – Self-care tips at the United States "National Diabetes Information Clearinghouse"