Megafauna
In zoology, megafauna (from Greek μέγας megas "large" and Neo-Latin fauna "animal life") are large animals. The most common thresholds to be a megafauna are weighing over 46 kilograms (100 lb)[1][2][3] (i.e., having a mass comparable to or larger than a human) or weighing over a tonne, 1,000 kilograms (2,205 lb)[1][4][5] (i.e., having a mass comparable to or larger than an ox). The first of these include many species not popularly thought of as overly large, and being the only few large animals left in a given range/area, such as white-tailed deer, Thomson's gazelle, and red kangaroo.
.jpg.webp)
In practice, the most common usage encountered in academic and popular writing describes land mammals roughly larger than a human that are not (solely) domesticated. The term is especially associated with the Pleistocene megafauna – the land animals often larger than their extant counterparts that are considered archetypical of the last ice age, such as mammoths, the majority of which in northern Eurasia and the Americas became extinct within the last forty thousand years.[6]
Among living animals, the term megafauna is most commonly used for the largest extant terrestrial mammals, which includes (but is not limited to) elephants, giraffes, hippopotamuses, rhinoceroses, and large bovines. Of these five categories of large herbivores, only bovines are presently found outside of Africa and southern Asia, but all the others were formerly more wide-ranging, with their ranges and populations continually shrinking and decreasing over time. Wild equines are another example of megafauna, but their current ranges are largely restricted to the Old World, specifically Africa and Asia. Megafaunal species may be categorized according to their dietary type: megaherbivores (e.g., elephants), megacarnivores (e.g., lions), and, more rarely, megaomnivores (e.g., bears). The megafauna is also categorized by the class of animals that it belongs to, which are mammals, birds, reptiles, amphibians, fish, and invertebrates.
Other common uses are for giant aquatic species, especially whales, as well as any of the larger wild or domesticated land animals such as larger antelope, deer, horse and cattle, as well as dinosaurs and other extinct giant reptiles.
The term megafauna is very rarely used to describe invertebrates, though it has occasionally been used for some species of invertebrates (which are on average much smaller than vertebrates) such as coconut crabs and Japanese spider crabs, as well as extinct invertebrates that were much larger than all similar invertebrate species alive today. One example are the meganisopterans, dragonfly-like insects from the Carboniferous period with wingspans reaching 1 m (3 ft).
Ecological strategy
Megafauna animals – in the sense of the largest mammals and birds – are generally K-strategists, with high longevity, slow population growth rates, low mortality rates, and (at least for the largest) few or no natural predators capable of killing adults.[7] These characteristics, although not exclusive to such megafauna, make them vulnerable to human overexploitation, in part because of their slow population recovery rates.[8][9]
Evolution of large body size
One observation that has been made about the evolution of larger body size is that rapid rates of increase that are often seen over relatively short time intervals are not sustainable over much longer time periods. In an examination of mammal body mass changes over time, the maximum increase possible in a given time interval was found to scale with the interval length raised to the 0.25 power.[10] This is thought to reflect the emergence, during a trend of increasing maximum body size, of a series of anatomical, physiological, environmental, genetic and other constraints that must be overcome by evolutionary innovations before further size increases are possible. A strikingly faster rate of change was found for large decreases in body mass, such as may be associated with the phenomenon of insular dwarfism. When normalized to generation length, the maximum rate of body mass decrease was found to be over 30 times greater than the maximum rate of body mass increase for a ten-fold change.[10]
In terrestrial mammals
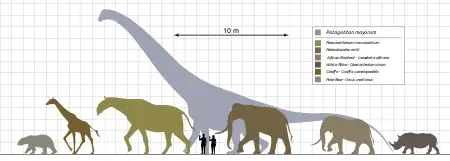
Subsequent to the Cretaceous–Paleogene extinction event that eliminated the non-avian dinosaurs about 66 Ma (million years) ago, terrestrial mammals underwent a nearly exponential increase in body size as they diversified to occupy the ecological niches left vacant.[11] Starting from just a few kg before the event, maximum size had reached ~50 kg a few million years later, and ~750 kg by the end of the Paleocene. This trend of increasing body mass appears to level off about 40 Ma ago (in the late Eocene), suggesting that physiological or ecological constraints had been reached, after an increase in body mass of over three orders of magnitude.[11] However, when considered from the standpoint of rate of size increase per generation, the exponential increase is found to have continued until the appearance of Indricotherium 30 Ma ago. (Since generation time scales with body mass0.259, increasing generation times with increasing size cause the log mass vs. time plot to curve downward from a linear fit.)[10]
Megaherbivores eventually attained a body mass of over 10,000 kg. The largest of these, indricotheres and proboscids, have been hindgut fermenters, which are believed to have an advantage over foregut fermenters in terms of being able to accelerate gastrointestinal transit in order to accommodate very large food intakes.[12] A similar trend emerges when rates of increase of maximum body mass per generation for different mammalian clades are compared (using rates averaged over macroevolutionary time scales). Among terrestrial mammals, the fastest rates of increase of body mass0.259 vs. time (in Ma) occurred in perissodactyls (a slope of 2.1), followed by rodents (1.2) and proboscids (1.1),[10] all of which are hindgut fermenters. The rate of increase for artiodactyls (0.74) was about a third that of perissodactyls. The rate for carnivorans (0.65) was slightly lower yet, while primates, perhaps constrained by their arboreal habits, had the lowest rate (0.39) among the mammalian groups studied.[10]
Terrestrial mammalian carnivores from several eutherian groups (the artiodactyl Andrewsarchus – formerly considered a mesonychid, the oxyaenid Sarkastodon, and the carnivorans Amphicyon and Arctodus) all reached a maximum size of about 1000 kg[11] (the carnivoran Arctotherium and the hyaenodontid Simbakubwa may have been somewhat larger). The largest known metatherian carnivore, Proborhyaena gigantea, apparently reached 600 kg, also close to this limit.[13] A similar theoretical maximum size for mammalian carnivores has been predicted based on the metabolic rate of mammals, the energetic cost of obtaining prey, and the maximum estimated rate coefficient of prey intake.[14] It has also been suggested that maximum size for mammalian carnivores is constrained by the stress the humerus can withstand at top running speed.[13]
Analysis of the variation of maximum body size over the last 40 Ma suggests that decreasing temperature and increasing continental land area are associated with increasing maximum body size. The former correlation would be consistent with Bergmann's rule,[15] and might be related to the thermoregulatory advantage of large body mass in cool climates,[11] better ability of larger organisms to cope with seasonality in food supply,[15] or other factors;[15] the latter correlation could be explained in terms of range and resource limitations.[11] However, the two parameters are interrelated (due to sea level drops accompanying increased glaciation), making the driver of the trends in maximum size more difficult to identify.[11]
In marine mammals

Since tetrapods (first reptiles, later mammals) returned to the sea in the Late Permian, they have dominated the top end of the marine body size range, due to the more efficient intake of oxygen possible using lungs.[16][17] The ancestors of cetaceans are believed to have been the semiaquatic pakicetids, no larger than dogs, of about 53 million years (Ma) ago.[18] By 40 Ma ago, cetaceans had attained a length of 20 m or more in Basilosaurus, an elongated, serpentine whale that differed from modern whales in many respects and was not ancestral to them. Following this, the evolution of large body size in cetaceans appears to have come to a temporary halt, and then to have backtracked, although the available fossil records are limited. However, in the period from 31 Ma ago (in the Oligocene) to the present, cetaceans underwent a significantly more rapid sustained increase in body mass (a rate of increase in body mass0.259 of a factor of 3.2 per million years) than achieved by any group of terrestrial mammals.[10] This trend led to the largest animal of all time, the modern blue whale. Several reasons for the more rapid evolution of large body size in cetaceans are possible. Fewer biomechanical constraints on increases in body size may be associated with suspension in water as opposed to standing against the force of gravity, and with swimming movements as opposed to terrestrial locomotion. Also, the greater heat capacity and thermal conductivity of water compared to air may increase the thermoregulatory advantage of large body size in marine endotherms, although diminishing returns apply.[10]
Among toothed whales, maximum body size appears to be limited by food availability. Larger size, as in sperm and beaked whales, facilitates deeper diving to access relatively easily-caught, large cephalopod prey in a less competitive environment. Compared to odontocetes, the efficiency of baleen whales' filter feeding scales more favorably with increasing size when planktonic food is dense, making larger size more advantageous. The lunge feeding technique of rorquals appears to be more energy efficient than the ram feeding of balaenid whales; the latter technique is used with less dense and patchy plankton.[19] The cooling trend in Earth's recent history may have generated more localities of high plankton abundance via wind-driven upwellings, facilitating the evolution of gigantic whales.[19]
Cetaceans are not the only marine mammals to reach tremendous sizes. The largest carnivorans of all time are marine pinnipeds, the largest of which is the southern elephant seal, which can reach more than 6 meters in length and weigh up to 5,000 kilograms (11,000 lb). Other large pinnipeds include the northern elephant seal at 4,000 kilograms (8,800 lb), walrus at 2,000 kilograms (4,400 lb), and Steller sea lion at 1,135 kilograms (2,502 lb). The sirenians are another group of marine mammals which adapted to fully aquatic life around the same time as the cetaceans did. Sirenians are closely related to elephants. The largest sirenian was the Steller's sea cow, which reached up to 10 meters in length and weighed 8,000 to 10,000 kilograms (18,000 to 22,000 lb), and was hunted to extinction in the 18th century. The semi-aquatic hippopotamus, which is the terrestrial mammal most closely related to cetaceans, can reach 3,200 kilograms (7,100 lb).
In flightless birds
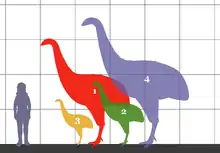
Because of the small initial size of all mammals following the extinction of the non-avian dinosaurs, nonmammalian vertebrates had a roughly ten-million-year-long window of opportunity (during the Paleocene) for evolution of gigantism without much competition.[20] During this interval, apex predator niches were often occupied by reptiles, such as terrestrial crocodilians (e.g. Pristichampsus), large snakes (e.g. Titanoboa) or varanid lizards, or by flightless birds[11] (e.g. Paleopsilopterus in South America). This is also the period when megafaunal flightless herbivorous gastornithid birds evolved in the Northern Hemisphere, while flightless paleognaths evolved to large size on Gondwanan land masses and Europe. Gastornithids and at least one lineage of flightless paleognath birds originated in Europe, both lineages dominating niches for large herbivores while mammals remained below 45 kg (in contrast with other landmasses like North America and Asia, which saw the earlier evolution of larger mammals) and were the largest European tetrapods in the Paleocene.[21]
Flightless paleognaths, termed ratites, have traditionally been viewed as representing a lineage separate from that of their small flighted relatives, the Neotropic tinamous. However, recent genetic studies have found that tinamous nest well within the ratite tree, and are the sister group of the extinct moa of New Zealand.[20][22][23] Similarly, the small kiwi of New Zealand have been found to be the sister group of the extinct elephant birds of Madagascar.[20] These findings indicate that flightlessness and gigantism arose independently multiple times among ratites via parallel evolution.
Predatory megafaunal flightless birds were often able to compete with mammals in the early Cenozoic. Later in the Cenozoic, however, they were displaced by advanced carnivorans and died out. In North America, the bathornithids Paracrax and Bathornis were apex predators but became extinct by the Early Miocene. In South America, the related phorusrhacids shared the dominant predatory niches with metatherian sparassodonts during most of the Cenozoic but declined and ultimately went extinct after eutherian predators arrived from North America (as part of the Great American Interchange) during the Pliocene. In contrast, large herbivorous flightless ratites have survived to the present.
However, none of the flightless birds of the Cenozoic, including the predatory Brontornis, possibly omnivorous Dromornis stirtoni[24] or herbivorous Vorombe, ever grew to masses much above 500 kg, and thus never attained the size of the largest mammalian carnivores, let alone that of the largest mammalian herbivores. It has been suggested that the increasing thickness of avian eggshells in proportion to egg mass with increasing egg size places an upper limit on the size of birds.[25][note 1] The largest species of Dromornis, D. stirtoni, may have gone extinct after it attained the maximum avian body mass and was then outcompeted by marsupial diprotodonts that evolved to sizes several times larger.[28]
In giant turtles
Giant tortoises were important components of late Cenozoic megafaunas, being present in every nonpolar continent until the arrival of homininans.[29][30] The largest known terrestrial tortoise was Megalochelys atlas, an animal that probably weighed about 1,000 kg.
Some earlier aquatic Testudines, e.g. the marine Archelon of the Cretaceous and freshwater Stupendemys of the Miocene, were considerably larger, weighing more than 2,000 kg.
Megafaunal mass extinctions
Timing and possible causes
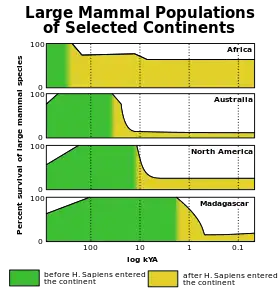

The Quaternary extinction event occurred during the latter half of the last ice age glacial period when many giant ice age mammals, such as woolly mammoths, went extinct in the Americas, Australia-New Guinea, and northern Eurasia. An analysis of the extinction event in North America found it to be unique among Cenozoic extinction pulses in its selectivity for large animals.[31]: Fig. 10 Various theories have attributed the wave of extinctions to human hunting, climate change, disease, extraterrestrial impact, or other causes. However, this extinction near the end of the Pleistocene was just one of a series of megafaunal extinction pulses that have occurred during the last 50,000 years over much of the Earth's surface, with Africa and southern Asia (where the local megafauna had a chance to evolve alongside modern humans) being comparatively less affected. The latter areas did suffer a gradual attrition of megafauna, particularly of the slower-moving species (a class of vulnerable megafauna epitomized by giant tortoises), over the last several million years.[32][33]
Outside the mainland of Afro-Eurasia, these megafaunal extinctions followed a highly distinctive landmass-by-landmass pattern that closely parallels the spread of humans into previously uninhabited regions of the world, and which shows no overall correlation with climatic history (which can be visualized with plots over recent geological time periods of climate markers such as marine oxygen isotopes or atmospheric carbon dioxide levels).[34][35] Australia[36] and nearby islands (e.g., Flores[37]) were struck first around 46,000 years ago, followed by Tasmania about 41,000 years ago (after formation of a land bridge to Australia about 43,000 years ago).[38][39][40] The role of humans in the extinction of Australia and New Guinea's megafauna has been disputed, with multiple studies showing a decline in the number of species prior to the arrival of humans on the continent and the absence of any evidence of human predation;[41][42][43][44] the impact of climate change has instead been cited for their decline.[45][41] Similarly, Japan lost most of its megafauna apparently about 30,000 years ago,[46] North America 13,000 years ago[note 2] and South America about 500 years later,[48][49] Cyprus 10,000 years ago,[50][51] the Antilles 6,000 years ago,[52][53] New Caledonia[54] and nearby islands[55] 3,000 years ago, Madagascar 2,000 years ago,[56] New Zealand 700 years ago,[57] the Mascarenes 400 years ago,[58] and the Commander Islands 250 years ago.[59] Nearly all of the world's isolated islands could furnish similar examples of extinctions occurring shortly after the arrival of humans, though most of these islands, such as the Hawaiian Islands, never had terrestrial megafauna, so their extinct fauna were smaller, but still displayed island gigantism.[34][35]
An analysis of the timing of Holarctic megafaunal extinctions and extirpations over the last 56,000 years has revealed a tendency for such events to cluster within interstadials, periods of abrupt warming, but only when humans were also present. Humans may have impeded processes of migration and recolonization that would otherwise have allowed the megafaunal species to adapt to the climate shift.[60] In at least some areas, interstadials were periods of expanding human populations.[61]
An analysis of Sporormiella fungal spores (which derive mainly from the dung of megaherbivores) in swamp sediment cores spanning the last 130,000 years from Lynch's Crater in Queensland, Australia, showed that the megafauna of that region virtually disappeared about 41,000 years ago, at a time when climate changes were minimal; the change was accompanied by an increase in charcoal, and was followed by a transition from rainforest to fire-tolerant sclerophyll vegetation. The high-resolution chronology of the changes supports the hypothesis that human hunting alone eliminated the megafauna, and that the subsequent change in flora was most likely a consequence of the elimination of browsers and an increase in fire.[62][63][64][65] The increase in fire lagged the disappearance of megafauna by about a century, and most likely resulted from accumulation of fuel once browsing stopped. Over the next several centuries grass increased; sclerophyll vegetation increased with a lag of another century, and a sclerophyll forest developed after about another thousand years.[64] During two periods of climate change about 120,000 and 75,000 years ago, sclerophyll vegetation had also increased at the site in response to a shift to cooler, drier conditions; neither of these episodes had a significant impact on megafaunal abundance.[64] Similar conclusions regarding the culpability of human hunters in the disappearance of Pleistocene megafauna were derived from high-resolution chronologies obtained via an analysis of a large collection of eggshell fragments of the flightless Australian bird Genyornis newtoni,[66][67][65] from analysis of Sporormiella fungal spores from a lake in eastern North America[68][69] and from study of deposits of Shasta ground sloth dung left in over half a dozen caves in the American Southwest.[70][71]
Continuing human hunting and environmental disturbance has led to additional megafaunal extinctions in the recent past, and has created a serious danger of further extinctions in the near future (see examples below). Direct killing by humans, primarily for meat or other body parts, is the most significant factor in contemporary megafaunal decline.[72][73]
A number of other mass extinctions occurred earlier in Earth's geologic history, in which some or all of the megafauna of the time also died out. Famously, in the Cretaceous–Paleogene extinction event the non-avian dinosaurs and most other giant reptiles were eliminated. However, the earlier mass extinctions were more global and not so selective for megafauna; i.e., many species of other types, including plants, marine invertebrates[74] and plankton, went extinct as well. Thus, the earlier events must have been caused by more generalized types of disturbances to the biosphere.
Effect on nutrient transport
Megafauna play a significant role in the lateral transport of mineral nutrients in an ecosystem, tending to translocate them from areas of high to those of lower abundance. They do so by their movement between the time they consume the nutrient and the time they release it through elimination (or, to a much lesser extent, through decomposition after death).[75] In South America's Amazon Basin, it is estimated that such lateral diffusion was reduced over 98% following the megafaunal extinctions that occurred roughly 12,500 years ago.[76][77] Given that phosphorus availability is thought to limit productivity in much of the region, the decrease in its transport from the western part of the basin and from floodplains (both of which derive their supply from the uplift of the Andes) to other areas is thought to have significantly impacted the region's ecology, and the effects may not yet have reached their limits.[77] In the sea, cetaceans and pinnipeds that feed at depth are thought to translocate nitrogen from deep to shallow water, enhancing ocean productivity, and counteracting the activity of zooplankton, which tend to do the opposite.[78]
Effect on methane emissions
Large populations of megaherbivores have the potential to contribute greatly to the atmospheric concentration of methane, which is an important greenhouse gas. Modern ruminant herbivores produce methane as a byproduct of foregut fermentation in digestion, and release it through belching or flatulence. Today, around 20% of annual methane emissions come from livestock methane release. In the Mesozoic, it has been estimated that sauropods could have emitted 520 million tons of methane to the atmosphere annually,[79] contributing to the warmer climate of the time (up to 10 °C warmer than at present).[79][80] This large emission follows from the enormous estimated biomass of sauropods, and because methane production of individual herbivores is believed to be almost proportional to their mass.[79]
Recent studies have indicated that the extinction of megafaunal herbivores may have caused a reduction in atmospheric methane. This hypothesis is relatively new.[81] One study examined the methane emissions from the bison that occupied the Great Plains of North America before contact with European settlers. The study estimated that the removal of the bison caused a decrease of as much as 2.2 million tons per year.[82] Another study examined the change in the methane concentration in the atmosphere at the end of the Pleistocene epoch after the extinction of megafauna in the Americas. After early humans migrated to the Americas about 13,000 BP, their hunting and other associated ecological impacts led to the extinction of many megafaunal species there. Calculations suggest that this extinction decreased methane production by about 9.6 million tons per year. This suggests that the absence of megafaunal methane emissions may have contributed to the abrupt climatic cooling at the onset of the Younger Dryas.[81] The decrease in atmospheric methane that occurred at that time, as recorded in ice cores, was 2–4 times more rapid than any other decrease in the last half million years, suggesting that an unusual mechanism was at work.[81]
Examples
The following are some notable examples of animals often considered as megafauna (in the sense of the "large animal" definition). This list is not intended to be exhaustive:
- Clade Synapsida
- Class Mammalia (phylogenetically, a clade within Therapsida; see below)
- Infraclass Metatheria
- Order Diprotodontia
- The red kangaroo (Osphranter rufus) is the largest living Australian mammal and marsupial at a weight of up to 85 kg (187 lb). However, its extinct relative, the giant short-faced kangaroo (Procoptodon goliah) reached 230 kg (510 lb),[83] while extinct diprotodonts attained the largest size of any marsupial in history, up to an estimated 2,750 kg (6,060 lb). The extinct marsupial lion (Thylacoleo carnifex), at up to 160 kg (350 lb) was the largest carnivorous marsupial ever known.
- Order Diprotodontia
- Infraclass Eutheria
- Superorder Afrotheria
- Order Proboscidea
- Elephants are the largest living land animals. They and their relatives arose in Africa, but until recently had a nearly worldwide distribution. The African bush elephant (Loxodonta africana) has a shoulder height of up to 4.3 m (14 ft) and weighs up to 10.4 tonnes (11.5 short tons).[84] Among recently extinct proboscideans, mammoths (Mammuthus) were close relatives of elephants, while mastodons (Mammut) were much more distantly related.
- Order Sirenia
- The largest extant sirenian at up to 1,500 kg (3,300 lb) is the West Indian manatee (Trichechus manatus). Steller's sea cow (Hydrodamalis gigas), which survived into modern times, was probably around five times as massive, but was exterminated by humans within 27 years of its discovery off the remote Commander Islands in 1741. In prehistoric times, this sea cow also lived along the coasts of northeastern Asia and northwestern North America; after already suffering a reduction in range from climate change thousands of years ago, it was apparently eliminated from these more accessible locations by the spread of aboriginal hunters.
- Order Proboscidea
- Superorder Xenarthra
- Order Cingulata
- The glyptodonts were a group of large, heavily armored ankylosaur-like xenarthrans related to living armadillos. They originated in South America, spread to North America during the Great American Interchange, and went extinct at the end of the Pleistocene epoch, presumably due to overhunting by humans.[85]
- Order Pilosa
- Ground sloths were another group of slow, terrestrial xenarthrans, related to modern tree sloths. They had a similar history, although they reached North America earlier, and spread farther north (e.g., Megalonyx). The largest genera, Megatherium and Eremotherium, reached sizes comparable to elephants.[85]
- Order Cingulata
- Superorder Euarchontoglires
- Order Primates
- The largest living primate, at up to 266 kg (586 lb), is the gorilla (Gorilla beringei and Gorilla gorilla, with three of four subspecies being critically endangered). The extinct Malagasy sloth lemur Archaeoindris reached a similar size, while the extinct Gigantopithecus blacki of Southeast Asia is believed to have been larger yet, although probably less than twice as large, contrary to early estimates (the absence of postcranial remains makes its size difficult to judge).[86] Some populations of archaic Homo were larger on average than recent Homo sapiens;[87][88] for example, Neanderthals were about 30% more massive.[89]
- Order Rodentia
- The extant capybara (Hydrochoerus hydrochaeris) of South America, the largest living rodent, weighs up to 80 kg (180 lb).[90] Several recently extinct North American forms were larger: the extinct capybara Neochoerus pinckneyi (another Neotropic migrant) was about 40% heavier on average; the giant beaver (Castoroides ohioensis) was similar in size. The extinct blunt-toothed giant hutia (Amblyrhiza inundata) of several Caribbean islands may have been larger still. However, several million years ago South America harbored much more massive rodents. Phoberomys pattersoni, known from a nearly full skeleton, probably reached 700 kg (1,500 lb). Fragmentary remains suggest that Josephoartigasia monesi grew to upwards of 1,000 kg (2,200 lb).
- Order Primates
- Superorder Laurasiatheria
- Order Carnivora
- The largest extant cats are in genus Panthera, including the tiger (P. tigris) and lion (P. leo).[91] The Siberian tiger should be the biggest wild cat according to Bergmann's rule, and has been regarded as such by some[92][93] but this is disputable.[94] Historically, wild Siberian tigers have declined in size, and they are now smaller than Bengal tigers (P. t. tigris);[95] however, Siberian tigers do still tend to be the largest of tigers in captivity, reaching about 320 kg (710 lb) in weight.[96] Panthera species are distinguished by morphological features which enable them to roar. Larger extinct cats include the American lion (P. atrox) and the South American saber-toothed cat (Smilodon populator).
- Bears are large carnivorans of the caniform suborder. The largest living forms are the polar bear (Ursus maritimus), with a body weight of up to 800 kg (1,800 lb),[97] and the nearly as large Kodiak bear (Ursus arctos middendorffi),[98] consistent with Bergmann's rule. Arctotherium augustans, an extinct short-faced bear from South America, was the largest predatory land mammal ever with an estimated average weight of 1,600 kg (3,500 lb).[99]
- Seals, sea lions, and walruses are amphibious marine carnivorans that evolved from bearlike ancestors. The southern elephant seal (Mirounga leonina) of Antarctic and subantarctic waters is the largest carnivoran known, with bull males reaching a maximum length of 6–7 m (20–23 ft) and maximum weight of 5,000 kg (11,000 lb).
- Order Perissodactyla
- Tapirs are browsing animals, with a short prehensile snout and pig-like form that appears to have changed little in 20 million years. They inhabit tropical forests of Southeast Asia and South and Central America, and include the largest surviving land animals of the latter two regions. There are four species.
- Rhinoceroses are odd-toed ungulates with horns made of keratin, the same type of protein composing hair. They are among the second-largest living land mammals at 850–3,800 kg. Three of five extant species are critically endangered, with some subspecies already extinct. Their extinct central Asian relatives, the indricotherines, were the largest terrestrial mammals of all time.
 Indian rhinoceros, from Dürer's woodcut
Indian rhinoceros, from Dürer's woodcut
- Order Artiodactyla
- Giraffes (Giraffa spp.) are the tallest living land animals, reaching heights of up to nearly 6 m (20 ft). The average weight is 1,192 kg (2,628 lb) for an adult male and 828 kg (1,825 lb) for an adult female with maximum weights of 1,930 kg (4,250 lb) and 1,180 kg (2,600 lb) recorded for males and females, respectively.
- Bovine ungulates include the largest surviving land animals of Europe and North America. The wild water buffalo (Bubalis arnee), bison (Bison bison and B. bonasus), and gaur (Bos gaurus) can all grow to weights of over 1,000 kg (2,200 lb).
- The semiaquatic hippopotamus (Hippopotamus amphibius) is the heaviest living member of the order Cetartiodactyla after the cetaceans. Mean adult weight is around 1,500 kg (3,300 lb) and 1,300 kg (2,900 lb) for males and females respectively, with large males reaching over 3,200 kg (7,100 lb). The hippopotamus and the much smaller endangered pygmy hippo (Choeropsis liberiensis) are believed to be the closest extant relatives of cetaceans. Hippopotamuses are among the megafaunal species most dangerous to humans, not only due to their size but also their fierce temperament.[100]
- Infraorder Cetacea
- Whales, dolphins, and porpoises are marine mammals. The blue whale (Balaenoptera musculus) is the largest baleen whale and the largest animal that has ever been known to live, at 30 metres (98 feet)[101] in length and 170 tonnes (190 short tons)[102] or more in weight. The sperm whale (Physeter macrocephalus) is the largest toothed whale and one of the largest predators in vertebrate history, as well as the planet's loudest and brainiest animal (with a brain about five times as massive as a human's). The killer whale (Orcinus orca) is the largest dolphin, reaching up to 9.8 metres (32 feet) and 10 tonnes.[103]
- Order Carnivora
- Superorder Afrotheria
- Infraclass Metatheria
- Class Mammalia (phylogenetically, a clade within Therapsida; see below)
- Clade Sauropsida
- Class Aves (phylogenetically, a clade within Coelurosauria, a taxon within the order Saurischia; see below)
- Infraclass Palaeognathae
- The ratites are an ancient and diverse group of flightless birds that are found on fragments of the former supercontinent Gondwana. The largest living bird, the ostrich (Struthio camelus) was surpassed by the extinct Vorombe of Madagascar, the heaviest of the group at up to (860 kg (1,900 lb)), and the extinct giant moa (Dinornis) of New Zealand, the tallest, growing to heights of 3.4 m (11 ft). The latter two are examples of island gigantism.
- Order Gastornithiformes
- Extinct dromornithids of Australia such as Dromornis approached the largest ratites in size. (Due to its small size for a continent and its isolation, Australia is sometimes viewed as the world's largest island; thus, these species could also be considered insular giants.)
- Order Cathartiformes
- The extinct condor-like teratorn Argentavis of South America had an estimated wing span of 5 to 6 m (16 to 20 ft) and a mass of approximately 70 kg (150 lb), making it the best example of a megafaunal flying bird.
- Infraclass Palaeognathae
- Class Reptilia (in the traditional paraphyletic semse)
- Order Crocodilia
- Alligators and crocodiles are large semiaquatic reptiles and are among the largest extant predators, the largest of which, the saltwater crocodile (Crocodylus porosus), reaches 6 m (20 ft) and can weigh up to 1,360 kg (3,000 lb), possibly up to 7 m (23 ft) in length and 2,000 kg (4,400 lb) in weight. Several other large species of crocodile such as the Nile crocodile, Orinoco crocodile and American crocodile may reach or exceed sizes of 6 m (20 ft) and weigh up to 1,000 kg (2,200 lb) or more. In the family Alligatoridae, the largest members are the black caiman and the American alligator, both of which can reach at least 5 m (16 ft), weighing up to 500 kg (1,100 lb), with unverified reports of sizes approaching 6 m (20 ft) and weights of over 1,000 kg (2,200 lb). Crocodilians' distant ancestors and their kin, the pseudosuchians (traditional crurotarsans), dominated the world in the late Triassic, until the Triassic–Jurassic extinction event allowed dinosaurs to overtake them. They remained diverse during the later Mesozoic, when crocodyliforms such as Deinosuchus and Sarcosuchus reached lengths of 12 m. Similarly large crocodilians, such as Mourasuchus and Purussaurus, were present as recently as the Miocene in South America.
- Order Squamata
- While the largest extant lizard, the Komodo dragon (Varanus komodoensis), another island giant, can reach 3 m (10 ft) in length, its extinct Australian relative Megalania may have reached more than twice that size. These monitor lizards' marine relatives, the mosasaurs, were apex predators in late Cretaceous seas.
- The heaviest extant snake is considered to be the green anaconda (Eunectes murinus), while the reticulated python (Python reticulatus), at up to 8.7 m or more, is considered the longest. An extinct Australian Pliocene species of Liasis, the Bluff Downs giant python, reached 10 m, while the Paleocene Titanoboa of South America reached lengths of 12–15 m and an estimated weight of about 1,135 kilograms (2,500 pounds).
- Order Testudines
- The largest extant turtle is the critically endangered marine leatherback turtle (Dermochelys coriacea), weighing up to 900 kg (2,000 lb). It is distinguished from other sea turtles by its lack of a bony shell. The largest known turtle was Archelon ischyros, a Late Cretaceous sea turtle up to 4.5 m (15 ft) long, 5.25 m (17 ft) wide between the tips of the front flippers, and estimated to have weighed over 2,200 kg (4,900 lb).[104] The most massive terrestrial chelonians are the giant tortoises of the Galápagos Islands (Chelonoidis niger) and Aldabra Atoll (Aldabrachelys gigantea), at up to 300 kg (660 lb). These tortoises are the biggest survivors of an assortment of giant tortoise species that were widely present on continental landmasses[29][30] and additional islands[29] during the Pleistocene.
- Order Crocodilia
- Class Aves (phylogenetically, a clade within Coelurosauria, a taxon within the order Saurischia; see below)
- Class Amphibia
- Order Urodela
- The Chinese Giant Salamander (Andrias davidianus) is the largest extant amphibian, reaching an average size of 1.8 m (6 ft).
- Order Urodela
- Class Actinopterygii
- Order Tetraodontiformes
- The largest extant bony fish is the ocean sunfish (Mola mola), whose average adult weight is 1,000 kg (2,200 lb). While phylogenetically a "bony fish", its skeleton is primarily cartilage (which is lighter than bone). It has a disk-shaped body, and propels itself with its long, thin dorsal and anal fins; it feeds primarily on jellyfish. In these three respects (as well as in its size and diving habits), it resembles a leatherback turtle.
- Order Lampriformes
- The giant oarfish (Regalecus glesne) is the longest bony fish, reaching 11 m (36 ft).
- Order Acipenseriformes
- The critically endangered beluga sturgeon (Huso huso) at up to 1,476 kg (3,254 lb) is the largest sturgeon (which are also mostly cartilaginous) and is considered the largest anadromous fish.
- Order Siluriformes
- The critically endangered Mekong giant catfish (Pangasianodon gigas), at up to 293 kg (646 lb), is often considered the largest freshwater fish.
- Order Tetraodontiformes
- Class Chondrichthyes
- Order Lamniformes
- The largest living predatory fish, the great white shark (Carcharodon carcharias), reaches weights up to 2,240 kg (4,940 lb). Its extinct relative O. megalodon was significantly larger, and is the largest predatory shark and/or fish of all time (and one of the largest predators in vertebrate history); it preyed on whales and other marine mammals.
- Order Orectolobiformes
- The largest extant shark, cartilaginous fish, and fish overall is the whale shark (Rhincodon typus), which reaches weights in excess of 21.5 tonnes (47,000 pounds). Like baleen whales, it is a filter feeder and primarily consumes plankton.
- Order Rajiformes
- Order Lamniformes
- Class Cephalopoda
- Order Teuthida
- A number of deep ocean creatures exhibit abyssal gigantism. These include the giant squid (Architeuthis) and colossal squid (Mesonychoteuthis hamiltoni); both (although rarely seen) are believed to attain lengths of 12 m (39 ft) or more. The latter is the world's largest invertebrate, and has the largest eyes of any animal. Both are preyed upon by sperm whales.
- Order Teuthida
Gallery
Pleistocene extinct megafauna

.jpg.webp) Diprotodon optatum was the largest known marsupial, approximately the size of a hippo. It became extinct 40,000 years ago.
Diprotodon optatum was the largest known marsupial, approximately the size of a hippo. It became extinct 40,000 years ago. "Megalania" (Varanus priscus), a giant carnivorous goanna of Australia, is the largest known terrestrial lizard; it might have grown to 7 meters long.
"Megalania" (Varanus priscus), a giant carnivorous goanna of Australia, is the largest known terrestrial lizard; it might have grown to 7 meters long..jpg.webp) Glyptodon, from the Pleistocene of South America, was a car-sized relative of armadillos.
Glyptodon, from the Pleistocene of South America, was a car-sized relative of armadillos..jpg.webp) Macrauchenia was the last and largest litoptern, an order of extinct South American native ungulates.[105][106]
Macrauchenia was the last and largest litoptern, an order of extinct South American native ungulates.[105][106] American lions (Panthera atrox) exceeded extant Old World lions in size and ranged over much of N. America until 11,000 BP.
American lions (Panthera atrox) exceeded extant Old World lions in size and ranged over much of N. America until 11,000 BP._-_Mauricio_Ant%C3%B3n.jpg.webp) Mammoths are iconic examples of megafauna, including the widespread woolly mammoth (pictured) and the even larger Columbian mammoth.
Mammoths are iconic examples of megafauna, including the widespread woolly mammoth (pictured) and the even larger Columbian mammoth. The subfossil lemur Archaeoindris was the largest lemur ever to exist, close in size to a modern gorilla.
The subfossil lemur Archaeoindris was the largest lemur ever to exist, close in size to a modern gorilla. Haast's eagle, the largest eagle ever known, attacking moa
Haast's eagle, the largest eagle ever known, attacking moa
Other extinct Cenozoic megafauna

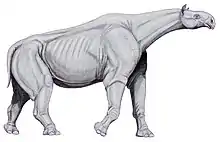 Asian indricothere and rhino relative Paraceratherium was among the largest land mammals,[107] about twice a bush elephant's mass.
Asian indricothere and rhino relative Paraceratherium was among the largest land mammals,[107] about twice a bush elephant's mass. Reconstructed jaws of megalodon (Otodus megalodon, the largest known shark and likely the largest known fish.
Reconstructed jaws of megalodon (Otodus megalodon, the largest known shark and likely the largest known fish.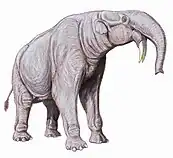 Deinotherium was a widespread Afro-Eurasian relative of elephants, with downward-curving tusks.
Deinotherium was a widespread Afro-Eurasian relative of elephants, with downward-curving tusks. Kelenken guillermoi was a terror bird and the tallest carnivorous bird ever, averaging a height of 3 m (9.8 ft) and exceeding 100 kg (220 lb) in mass.
Kelenken guillermoi was a terror bird and the tallest carnivorous bird ever, averaging a height of 3 m (9.8 ft) and exceeding 100 kg (220 lb) in mass. Gastornis gigantea (previously known as "Diatryma") was the largest bird to ever inhabit North America, weighing up to 225 kg (496 lb).
Gastornis gigantea (previously known as "Diatryma") was the largest bird to ever inhabit North America, weighing up to 225 kg (496 lb).
Extant

 The eastern gorilla is the largest and one of the most endangered primates on the planet.
The eastern gorilla is the largest and one of the most endangered primates on the planet.

 The critically endangered black rhinoceros, up to 3.75 metres (12.3 ft) long, is threatened by poaching.
The critically endangered black rhinoceros, up to 3.75 metres (12.3 ft) long, is threatened by poaching. Wild Bactrian camels are critically endangered. Their ancestors originated in North America.
Wild Bactrian camels are critically endangered. Their ancestors originated in North America.
 Hippopotamuses, the heaviest and most aquatic even-toed ungulates, are whales' closest living relatives.
Hippopotamuses, the heaviest and most aquatic even-toed ungulates, are whales' closest living relatives.
 The orca, the largest dolphin and pack predator, is highly intelligent and lives in complex societies.
The orca, the largest dolphin and pack predator, is highly intelligent and lives in complex societies.
 The common ostrich is the heaviest and tallest living bird weighing up to 156.8 kg (346 lb) and standing up to 2.8 m (9.2 ft) tall.
The common ostrich is the heaviest and tallest living bird weighing up to 156.8 kg (346 lb) and standing up to 2.8 m (9.2 ft) tall..jpg.webp)
 The Komodo dragon, an insular giant and the largest living lizard, has serrated teeth and a venomous bite.
The Komodo dragon, an insular giant and the largest living lizard, has serrated teeth and a venomous bite. The green anaconda, an aquatic constrictor, is the heaviest snake, weighing up to 97.5 kg (215 lb) or more.
The green anaconda, an aquatic constrictor, is the heaviest snake, weighing up to 97.5 kg (215 lb) or more.

 The great white, the largest macropredatory fish, is found worldwide.[108]
The great white, the largest macropredatory fish, is found worldwide.[108]

See also
- Australian megafauna
- Bergmann's rule
- Charismatic megafauna
- Cope's rule
- Deep-sea gigantism
- Fauna
- Island dwarfism
- Island gigantism
- Largest organisms
- Largest prehistoric animals
- List of heaviest land mammals
- List of largest mammals
- List of megafauna discovered in modern times
- Megafauna (mythology)
- Megafaunal wolf
- Megaflora
- Megaherb
- New World Pleistocene extinctions
- Pleistocene megafauna
- Quaternary extinction event
Notes
- Nonavian dinosaur size was not similarly constrained because they had a different relationship between body mass and egg size than birds. The 400 kg Aepyornis had larger eggs than nearly all dinosaurs.[26][27]
- Analysis indicates that 35 genera of North American mammals went extinct more or less simultaneously in this event.[47]
- Perspective makes the fish appear larger relative to the man standing behind it (another example of a megafaunal species) than it actually is.
References
- Stuart, A. J. (November 1991). "Mammalian extinctions in the Late Pleistocene of northern Eurasia and North America". Biological Reviews. 66 (4): 453–562. doi:10.1111/j.1469-185X.1991.tb01149.x. PMID 1801948. S2CID 41295526.
- Martin, P. S. (1984). "Prehistoric overkill: The global model". In Martin, P. S.; Klein, R. G. (eds.). Quaternary Extinctions: A Prehistoric Revolution. University of Arizona Press. pp. 354–403. ISBN 978-0-8165-1100-6. OCLC 258362030.
- Martin, P. S.; Steadman, D. W. (1999-06-30). "Prehistoric extinctions on islands and continents". In MacPhee, R. D. E (ed.). Extinctions in near time: causes, contexts and consequences. Advances in Vertebrate Paleobiology. Vol. 2. New York: Kluwer/Plenum. pp. 17–56. ISBN 978-0-306-46092-0. OCLC 41368299. Retrieved 2011-08-23. see page 17
- Richard A. Farina; Sergio F. Vizcaino; Gerry De Iuliis (2013). "The Great American Biotic Interchange". Megafauna: Giant Beasts of Pleistocene South America. Indiana University Press, Bloomington, Indiana. p. 150. ISBN 978-0-253-00230-3.
- Bernhard A. Huber; Bradley J. Sinclair; Karl-Heinz Lampe (2005). "Historical Determinants of Mammal Species in Africa". African Biodiversity: Molecules, Organisms, Ecosystems. Springer. p. 294. ISBN 978-0-387-24315-3.
- "Ice Age Animals". llinois State Museum. Retrieved 2023-09-23.
- https://www.britannica.com/science/K-selected-species. Britannica. Retrieved 2017-4-2.
- Barnosky, A. D. (2004-10-01). "Assessing the Causes of Late Pleistocene Extinctions on the Continents". Science. 306 (5693): 70–75. Bibcode:2004Sci...306...70B. CiteSeerX 10.1.1.574.332. doi:10.1126/science.1101476. PMID 15459379. S2CID 36156087.
- Brook, B. W.; Johnson, C. N. (2006). "Selective hunting of juveniles as a cause of the imperceptible overkill of the Australian Pleistocene megafauna". Alcheringa: An Australasian Journal of Palaeontology. 30 (sup1): 39–48. Bibcode:2006Alch...30S..39B. doi:10.1080/03115510609506854. S2CID 84205755.
- Evans, A. R.; Jones, D.; Boyer, A. G.; Brown, J. H.; Costa, D. P.; Ernest, S. K. M.; Fitzgerald, E. M. G.; Fortelius, M.; Gittleman, J. L.; Hamilton, M. J.; Harding, L. E.; Lintulaakso, K.; Lyons, S. K.; Okie, J. G.; Saarinen, J. J.; Sibly, R. M.; Smith, F. A.; Stephens, P. R.; Theodor, J. M.; Uhen, M. D. (2012-01-30). "The maximum rate of mammal evolution". PNAS. 109 (11): 4187–4190. Bibcode:2012PNAS..109.4187E. doi:10.1073/pnas.1120774109. PMC 3306709. PMID 22308461.
- Smith, F. A.; Boyer, A. G.; Brown, J. H.; Costa, D. P.; Dayan, T.; Ernest, S. K. M.; Evans, A. R.; Fortelius, M.; Gittleman, J. L.; Hamilton, M. J.; Harding, L. E.; Lintulaakso, K.; Lyons, S. K.; McCain, C.; Okie, J. G.; Saarinen, J. J.; Sibly, R. M.; Stephens, P. R.; Theodor, J.; Uhen, M. D. (2010-11-26). "The Evolution of Maximum Body Size of Terrestrial Mammals". Science. 330 (6008): 1216–1219. Bibcode:2010Sci...330.1216S. CiteSeerX 10.1.1.383.8581. doi:10.1126/science.1194830. PMID 21109666. S2CID 17272200.
- Clauss, M.; Frey, R.; Kiefer, B.; Lechner-Doll, M.; Loehlein, W.; Polster, C.; Roessner, G. E.; Streich, W. J. (2003-04-24). "The maximum attainable body size of herbivorous mammals: morphophysiological constraints on foregut, and adaptations of hindgut fermenters" (PDF). Oecologia. 136 (1): 14–27. Bibcode:2003Oecol.136...14C. doi:10.1007/s00442-003-1254-z. PMID 12712314. S2CID 206989975. Archived from the original (PDF) on 2019-06-08. Retrieved 2019-07-13.
- Sorkin, B. (2008-04-10). "A biomechanical constraint on body mass in terrestrial mammalian predators". Lethaia. 41 (4): 333–347. doi:10.1111/j.1502-3931.2007.00091.x.
- Carbone, C.; Teacher, A; Rowcliffe, J. M. (2007-01-16). "The Costs of Carnivory". PLOS Biology. 5 (2, e22): 363–368. doi:10.1371/journal.pbio.0050022. PMC 1769424. PMID 17227145.
- Ashton, K. G.; Tracy, M. C.; de Queiroz, A. (October 2000). "Is Bergmann's Rule Valid for Mammals?". The American Naturalist. 156 (4): 390–415. doi:10.1086/303400. JSTOR 10.1086/303400. PMID 29592141. S2CID 205983729.
- Webb, J. (2015-02-19). "Evolution 'favours bigger sea creatures'". BBC News. BBC. Retrieved 2015-02-22.
- Heim, N. A.; Knope, M. L.; Schaal, E. K.; Wang, S. C.; Payne, J. L. (2015-02-20). "Cope's rule in the evolution of marine animals". Science. 347 (6224): 867–870. Bibcode:2015Sci...347..867H. doi:10.1126/science.1260065. PMID 25700517.
- Thewissen, J. G. M.; Bajpai, S. (1 January 2001). "Whale Origins as a Poster Child for Macroevolution". BioScience. 51 (12): 1037–1049. doi:10.1641/0006-3568(2001)051[1037:WOAAPC]2.0.CO;2. ISSN 0006-3568.
- Goldbogen, J. A.; Cade, D. E.; Wisniewska, D. M.; Potvin, J.; Segre, P. S.; Savoca, M. S.; Hazen, E. L.; Czapanskiy, M. F.; Kahane-Rapport, S. R.; DeRuiter, S. L.; Gero, S.; Tønnesen, P.; Gough, W. T.; Hanson, M. B.; Holt, M. M.; Jensen, F. H.; Simon, M.; Stimpert, A. K.; Arranz, P.; Johnston, D. W.; Nowacek, D. P.; Parks, S. E.; Visser, F.; Friedlaender, A. S.; Tyack, P. L.; Madsen, P. T.; Pyenson, N. D. (2019). "Why whales are big but not bigger: Physiological drivers and ecological limits in the age of ocean giants". Science. 366 (6471): 1367–1372. Bibcode:2019Sci...366.1367G. doi:10.1126/science.aax9044. hdl:10023/19285. PMID 31831666. S2CID 209339266.
- Mitchell, K. J.; Llamas, B.; Soubrier, J.; Rawlence, N. J.; Worthy, T. H.; Wood, J.; Lee, M. S. Y.; Cooper, A. (2014-05-23). "Ancient DNA reveals elephant birds and kiwi are sister taxa and clarifies ratite bird evolution" (PDF). Science. 344 (6186): 898–900. Bibcode:2014Sci...344..898M. doi:10.1126/science.1251981. hdl:2328/35953. PMID 24855267. S2CID 206555952.
- Buffetaut, E.; Angst, D. (November 2014). "Stratigraphic distribution of large flightless birds in the Palaeogene of Europe and its palaeobiological and palaeogeographical implications". Earth-Science Reviews. 138: 394–408. Bibcode:2014ESRv..138..394B. doi:10.1016/j.earscirev.2014.07.001.
- Phillips MJ, Gibb GC, Crimp EA, Penny D (January 2010). "Tinamous and moa flock together: mitochondrial genome sequence analysis reveals independent losses of flight among ratites". Systematic Biology. 59 (1): 90–107. doi:10.1093/sysbio/syp079. PMID 20525622.
- Baker, A. J.; Haddrath, O.; McPherson, J. D.; Cloutier, A. (2014). "Genomic Support for a Moa-Tinamou Clade and Adaptive Morphological Convergence in Flightless Ratites". Molecular Biology and Evolution. 31 (7): 1686–1696. doi:10.1093/molbev/msu153. PMID 24825849.
- Murray, Peter F.; Vickers-Rich, Patricia (2004). Magnificent Mihirungs: The Colossal Flightless Birds of the Australian Dreamtime. Indiana University Press. pp. 51, 314. ISBN 978-0-253-34282-9. Retrieved 7 January 2012.
- Ibid (2004). p. 212. Indiana University Press. ISBN 978-0-253-34282-9.
- Kenneth Carpenter (1999). Eggs, Nests, and Baby Dinosaurs: A Look at Dinosaur Reproduction. Indiana University Press. p. 100. ISBN 978-0-253-33497-8. OCLC 42009424. Retrieved 6 May 2013.
- Jackson, F. D.; Varricchio, D. J.; Jackson, R. A.; Vila, B.; Chiappe, L. M. (2008). "Comparison of water vapor conductance in a titanosaur egg from the Upper Cretaceous of Argentina and a Megaloolithus siruguei egg from Spain". Paleobiology. 34 (2): 229–246. doi:10.1666/0094-8373(2008)034[0229:COWVCI]2.0.CO;2. ISSN 0094-8373. S2CID 85880201.
- Ibid (2004). p. 277. Indiana University Press. ISBN 978-0-253-34282-9.
- Hansen, D. M.; Donlan, C. J.; Griffiths, C. J.; Campbell, K. J. (April 2010). "Ecological history and latent conservation potential: large and giant tortoises as a model for taxon substitutions" (PDF). Ecography. 33 (2): 272–284. doi:10.1111/j.1600-0587.2010.06305.x. Archived from the original (PDF) on July 24, 2011. Retrieved 2011-02-26.
- Cione, A. L.; Tonni, E. P.; Soibelzon, L. (2003). "The Broken Zig-Zag: Late Cenozoic large mammal and tortoise extinction in South America". Rev. Mus. Argentino Cienc. Nat. Nueva Serie. 5 (1): 1–19. doi:10.22179/REVMACN.5.26. ISSN 1514-5158.
- Alroy, J. (1999), "Putting North America's End-Pleistocene Megafaunal Extinction in Context: Large-Scale Analyses of Spatial Patterns, Extinction Rates, and Size Distributions", in MacPhee, R. D. E. (ed.), Extinctions in Near Time: Causes, Contexts, and Consequences, Advances in Vertebrate Paleobiology, vol. 2, New York: Plenum, pp. 105–143, doi:10.1007/978-1-4757-5202-1_6, ISBN 978-1-4757-5202-1, OCLC 41368299
- Corlett, R. T. (2006). "Megafaunal extinctions in tropical Asia" (PDF). Tropinet. 17 (3): 1–3. Retrieved 2010-10-04.
- Edmeades, Baz. "Megafauna — First Victims of the Human-Caused Extinction". megafauna.com. (internet-published book with Foreword by Paul S. Martin). Archived from the original on 2014-12-25. Retrieved 2020-02-13.
- Martin, P. S. (2005). "Chapter 6. Deadly Syncopation". Twilight of the Mammoths: Ice Age Extinctions and the Rewilding of America. University of California Press. pp. 118–128. ISBN 978-0-520-23141-2. OCLC 58055404. Retrieved 2014-11-11.
- Burney, D. A.; Flannery, T. F. (July 2005). "Fifty millennia of catastrophic extinctions after human contact" (PDF). Trends in Ecology & Evolution. 20 (7): 395–401. doi:10.1016/j.tree.2005.04.022. PMID 16701402. Archived from the original (PDF) on 2010-06-10. Retrieved 2014-11-11.
- Roberts, R. G.; Flannery, T. F.; Ayliffe, L. K.; Yoshida, H.; Olley, J. M.; Prideaux, G. J.; Laslett, G. M.; Baynes, A.; Smith, M. A.; Jones, R.; Smith, B. L. (2001-06-08). "New Ages for the Last Australian Megafauna: Continent-Wide Extinction About 46,000 Years Ago" (PDF). Science. 292 (5523): 1888–1892. Bibcode:2001Sci...292.1888R. doi:10.1126/science.1060264. PMID 11397939. S2CID 45643228. Retrieved 2011-08-26.
- Callaway, E. (2016-09-21). "Human remains found in hobbit cave". Nature. doi:10.1038/nature.2016.20656. S2CID 89272546.
- Diamond, Jared (2008-08-13). "Palaeontology: The last giant kangaroo". Nature. 454 (7206): 835–836. Bibcode:2008Natur.454..835D. doi:10.1038/454835a. PMID 18704074. S2CID 36583693.
- Turney, C. S. M.; Flannery, T. F.; Roberts, R. G.; Reid, C.; Fifield, L. K.; Higham, T. F. G.; Jacobs, Z.; Kemp, N.; Colhoun, E. A.; Kalin, R. M.; Ogle, N. (2008-08-21). "Late-surviving megafauna in Tasmania, Australia, implicate human involvement in their extinction". PNAS. 105 (34): 12150–12153. Bibcode:2008PNAS..10512150T. doi:10.1073/pnas.0801360105. PMC 2527880. PMID 18719103.
- Roberts, R.; Jacobs, Z. (October 2008). "The Lost Giants of Tasmania" (PDF). Australasian Science. 29 (9): 14–17. Archived from the original (PDF) on 2011-09-27. Retrieved 2011-08-26.
- Field, Judith; Wroe, Stephen; Trueman, Clive N.; Garvey, Jillian; Wyatt-Spratt, Simon (2013-02-08). "Looking for the archaeological signature in Australian Megafaunal extinctions". Quaternary International. Peopling the last new worlds: the first colonisation of Sahul and the Americas. 285: 76–88. Bibcode:2013QuInt.285...76F. doi:10.1016/j.quaint.2011.04.013. ISSN 1040-6182.
- Dodson, John; Field, Judith H. (2018). "What does the occurrence of Sporormiella (Preussia) spores mean in Australian fossil sequences?". Journal of Quaternary Science. 33 (4): 380–392. Bibcode:2018JQS....33..380D. doi:10.1002/jqs.3020. ISSN 1099-1417. S2CID 133737405.
- Wroe, Stephen; Field, Judith H.; Archer, Michael; Grayson, Donald K.; Price, Gilbert J.; Louys, Julien; Faith, J. Tyler; Webb, Gregory E.; Davidson, Iain; Mooney, Scott D. (2013-09-03). "Reply to Brook et al: No empirical evidence for human overkill of megafauna in Sahul". Proceedings of the National Academy of Sciences. 110 (36): E3369. Bibcode:2013PNAS..110E3369W. doi:10.1073/pnas.1310440110. ISSN 0027-8424. PMC 3767508. PMID 24137797.
- Dortch, Joe; Cupper, Matt; Grün, Rainer; Harpley, Bernice; Lee, Kerrie; Field, Judith (2016-08-01). "The timing and cause of megafauna mass deaths at Lancefield Swamp, south-eastern Australia". Quaternary Science Reviews. 145: 161–182. Bibcode:2016QSRv..145..161D. doi:10.1016/j.quascirev.2016.05.042. ISSN 0277-3791.
- Wroe, Stephen; Field, Judith H.; Archer, Michael; Grayson, Donald K.; Price, Gilbert J.; Louys, Julien; Faith, J. Tyler; Webb, Gregory E.; Davidson, Iain; Mooney, Scott D. (2013-05-28). "Climate change frames debate over the extinction of megafauna in Sahul (Pleistocene Australia-New Guinea)". Proceedings of the National Academy of Sciences. 110 (22): 8777–8781. Bibcode:2013PNAS..110.8777W. doi:10.1073/pnas.1302698110. ISSN 0027-8424. PMC 3670326. PMID 23650401.
- Norton, C. J.; Kondo, Y.; Ono, A.; Zhang, Y.; Diab, M. C. (2009-05-23). "The nature of megafaunal extinctions during the MIS 3–2 transition in Japan". Quaternary International. 211 (1–2): 113–122. Bibcode:2010QuInt.211..113N. doi:10.1016/j.quaint.2009.05.002.
- Faith, J. T.; Surovell, T. A. (2009-12-08). "Synchronous extinction of North America's Pleistocene mammals". Proceedings of the National Academy of Sciences. 106 (49): 20641–20645. Bibcode:2009PNAS..10620641F. doi:10.1073/pnas.0908153106. PMC 2791611. PMID 19934040.
- Haynes, Gary (2009). "Introduction to the Volume". In Haynes, Gary (ed.). American Megafaunal Extinctions at the End of the Pleistocene. Vertebrate Paleobiology and Paleoanthropology. Springer. pp. 1–20. doi:10.1007/978-1-4020-8793-6_1. ISBN 978-1-4020-8792-9.
- Fiedel, Stuart (2009). "Sudden Deaths: The Chronology of Terminal Pleistocene Megafaunal Extinction". In Haynes, Gary (ed.). American Megafaunal Extinctions at the End of the Pleistocene. Vertebrate Paleobiology and Paleoanthropology. Springer. pp. 21–37. doi:10.1007/978-1-4020-8793-6_2. ISBN 978-1-4020-8792-9.
- Simmons, A. H. (1999). Faunal extinction in an island society: pygmy hippopotamus hunters of Cyprus. Interdisciplinary Contributions to Archaeology. Kluwer Academic/Plenum Publishers. p. 382. doi:10.1007/b109876. ISBN 978-0-306-46088-3. OCLC 41712246.
- Simmons, A. H.; Mandel, R. D. (December 2007). "Not Such a New Light: A Response to Ammerman and Noller". World Archaeology. 39 (4): 475–482. doi:10.1080/00438240701676169. JSTOR 40026143. S2CID 161791746.
- Steadman, D. W.; Martin, P. S.; MacPhee, R. D. E.; Jull, A. J. T.; McDonald, H. G.; Woods, C. A.; Iturralde-Vinent, M.; Hodgins, G. W. L. (2005-08-16). "Asynchronous extinction of late Quaternary sloths on continents and islands". Proc. Natl. Acad. Sci. USA. 102 (33): 11763–11768. Bibcode:2005PNAS..10211763S. doi:10.1073/pnas.0502777102. PMC 1187974. PMID 16085711.
- Cooke, S. B.; Dávalos, L. M.; Mychajliw, A. M.; Turvey, S. T.; Upham, N. S. (2017). "Anthropogenic Extinction Dominates Holocene Declines of West Indian Mammals". Annual Review of Ecology, Evolution, and Systematics. 48 (1): 301–327. doi:10.1146/annurev-ecolsys-110316-022754. S2CID 90558542.
- Anderson, A.; Sand, C.; Petchey, F.; Worthy, T. H. (2010). "Faunal extinction and human habitation in New Caledonia: Initial results and implications of new research at the Pindai Caves". Journal of Pacific Archaeology. 1 (1): 89–109. hdl:10289/5404.
- White, A. W.; Worthy, T. H.; Hawkins, S.; Bedford, S.; Spriggs, M. (2010-08-16). "Megafaunal meiolaniid horned turtles survived until early human settlement in Vanuatu, Southwest Pacific". Proc. Natl. Acad. Sci. USA. 107 (35): 15512–15516. Bibcode:2010PNAS..10715512W. doi:10.1073/pnas.1005780107. PMC 2932593. PMID 20713711.
- Burney, D. A.; Burney, L. P.; Godfrey, L. R.; Jungers, W. L.; Goodman, S. M.; Wright, H. T.; Jull. A. J. T. (July 2004). "A chronology for late prehistoric Madagascar". Journal of Human Evolution. 47 (1–2): 25–63. doi:10.1016/j.jhevol.2004.05.005. PMID 15288523.
- Holdaway, R. N.; Jacomb, C. (2000-03-24). "Rapid Extinction of the Moas (Aves: Dinornithiformes): Model, Test, and Implications". Science. 287 (5461): 2250–2254. Bibcode:2000Sci...287.2250H. doi:10.1126/science.287.5461.2250. PMID 10731144.
- Janoo, A. (April 2005). "Discovery of isolated dodo bones (Raphus cucullatus (L.), Aves, Columbiformes) from Mauritius cave shelters highlights human predation, with a comment on the status of the family Raphidae Wetmore, 1930". Annales de Paléontologie. 91 (2): 167–180. Bibcode:2005AnPal..91..167J. doi:10.1016/j.annpal.2004.12.002.
- Anderson, P. K. (July 1995). "Competition, Predation, and the Evolution and Extinction of Steller's Sea Cow, Hydrodamalis gigas". Marine Mammal Science. 11 (3): 391–394. doi:10.1111/j.1748-7692.1995.tb00294.x. Archived from the original on 2011-05-11. Retrieved 2011-08-30.
- Cooper, A.; Turney, C.; Hughen, K. A.; Brook, B. W.; McDonald, H. G.; Bradshaw, C. J. A. (2015-07-23). "Abrupt warming events drove Late Pleistocene Holarctic megafaunal turnover". Science. 349 (6248): 602–6. Bibcode:2015Sci...349..602C. doi:10.1126/science.aac4315. PMID 26250679. S2CID 31686497.
- Müller, U. C.; Pross, J.; Tzedakis, P. C.; Gamble, C.; Kotthoff, U.; Schmiedl, G.; Wulf, S.; Christanis, K. (February 2011). "The role of climate in the spread of modern humans into Europe". Quaternary Science Reviews. 30 (3–4): 273–279. Bibcode:2011QSRv...30..273M. doi:10.1016/j.quascirev.2010.11.016.
- Biello, D. (2012-03-22). "Big Kill, Not Big Chill, Finished Off Giant Kangaroos". Scientific American news. Retrieved 2012-03-25.
- McGlone, M. (2012-03-23). "The Hunters Did It". Science. 335 (6075): 1452–1453. Bibcode:2012Sci...335.1452M. doi:10.1126/science.1220176. PMID 22442471. S2CID 36914192.
- Rule, S.; Brook, B. W.; Haberle, S. G.; Turney, C. S. M.; Kershaw, A. P. (2012-03-23). "The Aftermath of Megafaunal Extinction: Ecosystem Transformation in Pleistocene Australia". Science. 335 (6075): 1483–1486. Bibcode:2012Sci...335.1483R. doi:10.1126/science.1214261. PMID 22442481. S2CID 26675232.
- Johnson, C. N.; Alroy, J.; Beeton, N. J.; Bird, M. I.; Brook, B. W.; Cooper, A.; Gillespie, R.; Herrando-Pérez, S.; Jacobs, Z.; Miller, G. H.; Prideaux, G. J.; Roberts, R. G.; Rodríguez-Rey, M.; Saltré, F.; Turney, C. S. M.; Bradshaw, C. J. A. (10 February 2016). "What caused extinction of the Pleistocene megafauna of Sahul?". Proceedings of the Royal Society B: Biological Sciences. 283 (1824): 20152399. doi:10.1098/rspb.2015.2399. PMC 4760161. PMID 26865301.
- Miller, G. H.; Magee, J. W.; Johnson, B. J.; Fogel, M. L.; Spooner, N. A.; McCulloch, M. T.; Ayliffe, L. K. (1999-01-08). "Pleistocene Extinction of Genyornis newtoni: Human Impact on Australian Megafauna". Science. 283 (5399): 205–208. doi:10.1126/science.283.5399.205. PMID 9880249.
- Miller, G.; Magee, J.; Smith, M.; Spooner, N.; Baynes, A.; Lehman, S.; Fogel, M.; Johnston, H.; Williams, D.; Clark, P.; Florian, C.; Holst, R.; DeVogel, S. (2016-01-29). "Human predation contributed to the extinction of the Australian megafaunal bird Genyornis newtoni ~47 ka". Nature Communications. 7: 10496. Bibcode:2016NatCo...710496M. doi:10.1038/ncomms10496. PMC 4740177. PMID 26823193.
- Johnson, C. (2009-11-20). "Megafaunal Decline and Fall". Science. 326 (5956): 1072–1073. Bibcode:2009Sci...326.1072J. doi:10.1126/science.1182770. PMID 19965418. S2CID 206523763.
- Gill, J. L.; Williams, J. W.; Jackson, S. T.; Lininger, K. B.; Robinson, G. S. (2009-11-20). "Pleistocene Megafaunal Collapse, Novel Plant Communities, and Enhanced Fire Regimes in North America" (PDF). Science. 326 (5956): 1100–1103. Bibcode:2009Sci...326.1100G. doi:10.1126/science.1179504. PMID 19965426. S2CID 206522597.
- Fiedal, Stuart (2009). "Sudden Deaths: The Chronology of Terminal Pleistocene Megafaunal Extinction". In Haynes, Gary (ed.). American Megafaunal Extinctions at the End of the Pleistocene. Vertebrate Paleobiology and Paleoanthropology. Springer. pp. 21–37. doi:10.1007/978-1-4020-8793-6_2. ISBN 978-1-4020-8792-9.
- Martin, P. S. (2005). "Chapter 4. Ground Sloths at Home". Twilight of the Mammoths: Ice Age Extinctions and the Rewilding of America. University of California Press. pp. 78–99. ISBN 978-0-520-23141-2. OCLC 58055404. Retrieved 2014-11-11.
- Milman, Oliver (February 6, 2019). "The killing of large species is pushing them towards extinction, study finds". The Guardian. Retrieved February 13, 2019.
- Ripple, W. J.; et al. (2019). "Are we eating the world's megafauna to extinction?". Conservation Letters. 12 (3): e12627. doi:10.1111/conl.12627.
- Alroy, J. (2008-08-12). "Dynamics of origination and extinction in the marine fossil record". PNAS. 105 Suppl 1 (Supplement_1): 11536–11542. Bibcode:2008PNAS..10511536A. doi:10.1073/pnas.0802597105. PMC 2556405. PMID 18695240.
- Wolf, A.; Doughty, C. E.; Malhi, Y. (2013). "Lateral Diffusion of Nutrients by Mammalian Herbivores in Terrestrial Ecosystems". PLoS ONE. 8 (8): e71352. Bibcode:2013PLoSO...871352W. doi:10.1371/journal.pone.0071352. PMC 3739793. PMID 23951141.
- Marshall, M. (2013-08-11). "Ecosystems still feel the pain of ancient extinctions". New Scientist. Retrieved 2013-08-12.
- Doughty, C. E.; Wolf, A.; Malhi, Y. (2013-08-11). "The legacy of the Pleistocene megafauna extinctions on nutrient availability in Amazonia". Nature Geoscience. 6 (9): 761–764. Bibcode:2013NatGe...6..761D. doi:10.1038/ngeo1895.
- Roman, J.; McCarthy, J.J. (2010). "The Whale Pump: Marine Mammals Enhance Primary Productivity in a Coastal Basin". PLOS ONE. 5 (10): e13255. Bibcode:2010PLoSO...513255R. doi:10.1371/journal.pone.0013255. PMC 2952594. PMID 20949007.
- Wilkinson, D. M.; Nisbet, E. G.; Ruxton, G. D. (2012-05-08). "Could methane produced by sauropod dinosaurs have helped drive Mesozoic climate warmth?". Current Biology. 22 (9): R292–R293. doi:10.1016/j.cub.2012.03.042. PMID 22575462.
- "Dinosaur gases 'warmed the Earth'". BBC Nature News. 2012-05-07. Retrieved 2012-05-08.
- Smith, F. A.; Elliot, S. M.; Lyons, S. K. (2010-05-23). "Methane emissions from extinct megafauna". Nature Geoscience. 3 (6): 374–375. Bibcode:2010NatGe...3..374S. doi:10.1038/ngeo877.
- Kelliher, F. M.; Clark, H. (2010-03-15). "Methane emissions from bison—An historic herd estimate for the North American Great Plains". Agricultural and Forest Meteorology. 150 (3): 473–577. Bibcode:2010AgFM..150..473K. doi:10.1016/j.agrformet.2009.11.019.
- Helgen, Kristofer M.; et al. (2006). "Ecological and evolutionary significance of sizes of giant extinct kangaroos" (PDF). Australian Journal of Zoology. 54 (4): 293–301. doi:10.1071/ZO05077 – via si.edu.
- Larramendi, A. (2016). "Shoulder height, body mass and shape of proboscideans" (PDF). Acta Palaeontologica Polonica. 61 (3): 537–574. doi:10.4202/app.00136.2014. S2CID 2092950. Retrieved 2018-03-22.
- Fariña, Richard A.; Vizcaíno, Sergio F.; De Iuliis, Gerry (22 May 2013). Megafauna: Giant Beasts of Pleistocene South America. Indiana University Press. ISBN 978-0-253-00719-3. OCLC 779244424.
- Zhang, Y.; Harrison, T. (2017). "Gigantopithecus blacki: a giant ape from the Pleistocene of Asia revisited". American Journal of Physical Anthropology. 162 (S63): 153–177. doi:10.1002/ajpa.23150. PMID 28105715.
- Ruff, C. B.; Trinkaus, E.; Holliday, T. W. (1997-05-08). "Body mass and encephalization in Pleistocene Homo". Nature. 387 (6629): 173–176. Bibcode:1997Natur.387..173R. doi:10.1038/387173a0. PMID 9144286. S2CID 4320413.
- Grine, F. E.; Jumgers, W. L.; Tobias, P. V.; Pearson, O. M. (June 1995). "Fossil Homo femur from Berg Aukas, northern Namibia". American Journal of Physical Anthropology. 97 (2): 151–185. doi:10.1002/ajpa.1330970207. PMID 7653506.
- Kappelman, John (1997-05-08). "They might be giants". Nature. 387 (6629): 126–127. Bibcode:1997Natur.387..126K. doi:10.1038/387126a0. PMID 9144276. S2CID 4328242.
- de Barros Ferraz, K.M.P.M.; Bonach, K.; Verdade, L.M. (2005). "Relationship between body mass and body length in capybaras (Hydrochoerus hydrochaeris)". Biota Neotropica. 5 (1): 197–200. doi:10.1590/S1676-06032005000100020.
- Kitchener, A.C.; Breitenmoser-Würsten, C.; Eizirik, E.; Gentry, A.; Werdelin, L.; Wilting, A.; Yamaguchi, N. (2017). "A revised taxonomy of the Felidae: The final report of the Cat Classification Task Force of the IUCN Cat Specialist Group" (PDF). Cat News (Special Issue 11).
- Brakefield, Tom (1993). Big Cats: Kingdom of Might. Voyageur Press. p. 44. ISBN 978-0-89658-329-0.
- Nowell, Kristin; Jackson, Peter (1996). Wild Cats: Status Survey and Conservation Action Plan (PDF). Gland, Switzerland: IUCN/SSC Cat Specialist Group. p. 56. ISBN 978-2-8317-0045-8.
- Kitchener, A.; Yamaguchi, N. (2009). "What is a Tiger? Biogeography, Morphology, and Taxonomy". In Tilson, R.; Nyhus, P. J. (eds.). Tigers of the World: The Science, Politics and Conservation of Panthera tigris. Academic Press. pp. 53–84. ISBN 978-0-08-094751-8.
- Slaght, J. C., Miquelle, D. G., Nikolaev, I. G., Goodrich, J. M., Smirnov, E. N., Traylor-Holzer, K., Christie, S., Arjanova, T., Smith, J. L. D. and Karanth, K. U. (2005). "Chapter 6. Who's king of the beasts? Historical and contemporary data on the body weight of wild and captive Amur tigers in comparison with other subspecies" (PDF). In D. G. Miquelle; E. N. Smirnov; J.M. Goodrich (eds.). Tigers in Sikhote-Alin Zapovednik: Ecology and Conservation (in Russian). Vladivostok, Russia: PSP. pp. 25–35.
{{cite book}}: CS1 maint: multiple names: authors list (link) - "Samson - the Biggest Tiger".
- DeMaster, D.P.; Stirling, I. (8 May 1981). "Ursus maritimus". Mammalian Species (145): 1–7. doi:10.2307/3504138. JSTOR 3503828.
- Pasitschniak-Arts, M. (23 April 1993). "Ursus arctos". Mammalian Species (439): 1–10. doi:10.2307/3503828. JSTOR 3504138.
- Soibelzon, L. H.; Schubert, B. W. (January 2011). "The Largest Known Bear, Arctotherium angustidens, from the Early Pleistocene Pampean Region of Argentina: With a Discussion of Size and Diet Trends in Bears". Journal of Paleontology. 85 (1): 69–75. doi:10.1666/10-037.1. hdl:11336/104215. S2CID 129585554. Retrieved 2011-06-01.
- Swift, E. M. (1997-11-17). "What Big Mouths They Have: Travelers in Africa who run afoul of hippos may not live to tell the tale". Sports Illustrated Vault. Time Inc. Retrieved 2011-11-16.
- ^ J. Calambokidis and G. Steiger (1998). Blue Whales. Voyageur Press. ISBN 0-89658-338-4.
- ^ "Animal Records". Smithsonian National Zoological Park. Retrieved 2007-05-29.
- "Killer Whale (Orca) Facts and Information". SeaWorld Parks & Entertainment. Retrieved 31 August 2023.
- "The Archelon". Black Hills Institute of Geological Research. Archived from the original on March 12, 2016. Retrieved 23 December 2018.
- Palmer, D., ed. (1999). The Marshall Illustrated Encyclopedia of Dinosaurs and Prehistoric Animals. London: Marshall Editions. p. 248. ISBN 978-1-84028-152-1.
- Moyano, S.R.; Giannini, N.P. (2018-10-10). "Cranial characters associated with the proboscis postnatal-development in Tapirus (Perissodactyla: Tapiridae) and comparisons with other extant and fossil hoofed mammals". Zoologischer Anzeiger. 277 (7554): 143–147. doi:10.1016/j.jcz.2018.08.005. ISSN 0044-5231. S2CID 92143497.
- Tsubamoto, T. (2012). "Estimating body mass from the astragalus in mammals". Acta Palaeontologica Polonica: 259–265. doi:10.4202/app.2011.0067. S2CID 54686160.
- Sample, Ian (19 February 2010). "Great white shark is more endangered than tiger, claims scientist". The Guardian. Retrieved 14 August 2013.