Melam (chemistry)
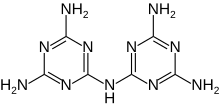
Melam (N2-(4,6-diamino-1,3,5-triazin-2-yl)-1,3,5-triazine-2,4,6-triamine) is a condensation product of melamine.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
N2-(4,6-Diamino-1,3,5-triazin-2-yl)-1,3,5-triazine-2,4,6-triamine | |
| Other names
A1,3,5-Triazine-2,4,6-triamine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.020.632 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H9N11 | |
| Molar mass | 235.21 g/mol |
| Appearance | white powder |
| insoluble | |
| Solubility | slightly soluble in acids |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Synthesis
Melam was discovered by Liebig in 1834 from the residue of heating ammonium thiocyanate.
Chemical property
In the presence of 30% ammonia, melam undergoes hydrolysis to form ammeline and melamine. It also reacts with concentrated nitric acid, producing cyanuric acid.
Upon heating, melam first loses ammonia to form melem, and then melon.
References
- B. Bann and S.A. Miller, "Melamines and derivatives of melamine", Chemical Reviews, vol.58, p131-172 (1958).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.