Mercury(II) reductase
Mercury(II) reductase (EC 1.16.1.1), commonly known as MerA, is an oxidoreductase enzyme and flavoprotein that catalyzes the reduction of Hg2+ to Hg0. Mercury(II) reductase is found in the cytoplasm of many eubacteria[1] in both aerobic and anaerobic environments[2] and serves to convert toxic mercury ions into relatively inert elemental mercury.
| mercury (II) reductase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| EC no. | 1.16.1.1 | ||||||||
| CAS no. | 67880-93-7 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Gene
Mercury(II) reductase, commonly known as MerA, is encoded in a structural gene found on the mer loci or as transposon 501 (Tn501).[3] It shares the same promoter region as mercury transport class proteins, such as MerP and MerT, and regulatory factor MerD.[1] MerA transcription is regulated by both MerR and MerD.[1]
Function
Free mercury ions can bind to metalloproteins, particularly those with cysteine residues, and can cause incorrect conformations resulting in function loss. This can cause death in many bacteria, as can many other heavy metals, and thus, needs to be removed from the cell or transformed into a chemically inert form. Mercury(II) reductase takes Hg2+ and catalyzes its reduction into Hg0 which is then released from the cell as a vapour. Mercury in its elemental form does not have the ability to form stable complexes with amino acid residues in proteins so is less dangerous than its ionic form.
Mechanism
Hg2+ + NADPH → Hg0 + H+ + NADP+
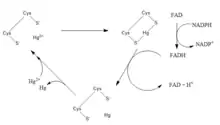
1. Hg2+ + 2Cys-S− → Cys-S-Hg-S-Cys
2. FAD + NADPH → FADH− + NADP+
3. Cys-S-Hg-S-Cys + FADH− → H+ + Hg0 + FAD + 2Cys-S−
The substrates used in mercuric(II) reductase, as shown above, are Hg2+ and NADPH. In the catalytic active site of the enzyme, Hg2+ is held as a complex with two cysteine thiolates in a linear geometry.[4] NADPH from the cytoplasm of the cell undergo a hydride transfer with an embedded FAD forming FADH−.[4] The resulting FADH− then reduces Hg2+ into Hg0, in turn being oxidized back into FAD.[4] After reduction, the mercury is then released from the enzyme as a volatile vapour.
Mercury(II) reductase cannot completely reduce organomercury compounds such as methyl mercury. Thus, MerB cleaves the carbon-mercury bonds via protonolysis and forms a mercury dithiolate complex, upon which MerB transports the mercury directly to MerA for reduction.[5]
Structure
The active form of mercury(II) reductase is found as a homodimer.[4] It has a quaternary conformation and the monomer is composed of two domains.[4]
NmerA
One of the domains of mercuric reductase, NmerA, has a structural fold of βαββαβ.[6] It is attached to the active site through linkers made of around 30 amino acids.[6] NmerA contains two cysteine residues which function in the acquisition of Hg2+ from other proteins or inorganic ligands such as MerT and direct transport to the catalytic active site of MerA.[7] Very few mercuric(II) reductases have been found to lack the NmerA domain.[6]
Active site
The active site of MerA consists of four cysteine residues, a FAD, and a tyrosine residue. When bound to a Hg2+, a complex is formed with at least two cysteine thiolates at any time until release.[4] Two cysteine residues (Cys-136 and Cys-141) are buried within the protein and the other two cysteine residues (Cys-558' and Cys-559') are found near the surface near the C terminus.[4] The buried cysteine residues function as the site of catalysis whereas the surface cysteine residues function as transport to the site of catalysis.[4]
During Hg2+ transfer to the catalytic active site from the C terminus cysteine residues, a trigonal planar intermediate is formed stabilized by hydrogen bonding of a water molecule to the thiolates.[4] The water molecule is held in place by hydrogen bonding from the hydroxyl group of a nearby tyrosine residue (Tyr-194).[4]
Mercury transport
Various proteins assist in transporting mercury to mercury(II) reductase. MerP, a periplasmic mercury transport protein found in gram negative bacteria, transports mercury through the outer membrane into the inner membrane where it holds the mercury for another protein to bind to it and transport it to mercury(II) reductase.[1] MerT, a membrane bound protein found in both gram negative and gram positive bacteria, binds to free floating mercury.[1] Mercury(II) reductase can directly take mercury from MerT and MerP.[1]
When mercury enters the cell and is not bound to a membrane protein, mercury(II) reductase can transport it to its active site on its own depending on the size of its ligands.[8] If the ligands attached to mercury are large, mercury(II) reductase uses the C-terminus cysteine residues to transport the mercury to its active site.[8] If the ligands are small, mercury can go directly the active site for reduction.[8] The ligands can be removed by the NmerA domain.[8]
In the case of organomercury compounds, MerB breaks the Hg-C bonds and transports the Hg to mercuric(II) reductase.[5]
Regulation
When not bound to Hg2+, mercury(II) reductase acts as an oxidase creating toxic hydrogen peroxide.[1] Thus, excess of the enzyme can result in bacterial death. Bacteria developed two regulatory proteins, MerR and MerD, for mercury(II) reductase.[1] There are two promoter regions on the mer loci: The first region encodes regulator protein MerR, and the second region encodes the structural mer genes and the gene for the regulatory protein MerD. Both promoter regions overlap.[1]
MerR binds to an operator in the structural mer gene promoter called MerO.[1] This binding causes the DNA of the mer loci to bend to where RNA polymerase can not read the region. However, Hg2+ can bind to MerR and allosterically change the shape of the DNA, so that RNA polymerase can read the promoter region of the structural genes.[1] Since both promoter regions overlap when apoMerR is bound to MerO, the change in DNA conformation causes neither the structural genes nor the regulatory genes to be read. This makes MerR a negative autoregulator.[1]
MerR forms a stable trigonal planar complex with Hg2+, which causes it to be released much later than when mercury(II) reductase has reduced all free Hg2+ in the cytoplasm.[1] Thus, it causes an excess in production of mercuric(II) reductase. To circumvent this problem, MerD also binds to MerO in order to act antagonistically to Hg2+ bound MerR.[1] MerD is produced when MerR is active with Hg2+ since MerD is encoded in the structural mer genes.
Applications and uses
In waste-water treatment procedures, mercury is sometimes removed from the water by making the water flow through a biofilm rich with mercury(II) reductase-containing bacteria.[1]
References
- Barkay T, Miller SM, Summers AO (June 2003). "Bacterial mercury resistance from atoms to ecosystems". FEMS Microbiology Reviews. 27 (2–3): 355–84. doi:10.1016/s0168-6445(03)00046-9. PMID 12829275.
- Ní Chadhain SM, Schaefer JK, Crane S, Zylstra GJ, Barkay T (October 2006). "Analysis of mercuric reductase (merA) gene diversity in an anaerobic mercury-contaminated sediment enrichment". Environmental Microbiology. 8 (10): 1746–52. doi:10.1111/j.1462-2920.2006.01114.x. PMID 16958755.
- Moore MJ, Walsh CT (February 1989). "Mutagenesis of the N- and C-terminal cysteine pairs of Tn501 mercuric ion reductase: consequences for bacterial detoxification of mercurials". Biochemistry. 28 (3): 1183–94. doi:10.1021/bi00429a036. PMID 2540817.
- Lian P, Guo HB, Riccardi D, Dong A, Parks JM, Xu Q, Pai EF, Miller SM, Wei DQ, Smith JC, Guo H (November 2014). "X-ray structure of a Hg2+ complex of mercuric reductase (MerA) and quantum mechanical/molecular mechanical study of Hg2+ transfer between the C-terminal and buried catalytic site cysteine pairs". Biochemistry. 53 (46): 7211–22. doi:10.1021/bi500608u. PMC 4245977. PMID 25343681.
- Lafrance-Vanasse J, Lefebvre M, Di Lello P, Sygusch J, Omichinski JG (January 2009). "Crystal structures of the organomercurial lyase MerB in its free and mercury-bound forms: insights into the mechanism of methylmercury degradation". The Journal of Biological Chemistry. 284 (2): 938–44. doi:10.1074/jbc.m807143200. PMID 19004822.
- Hong L, Sharp MA, Poblete S, Biehl R, Zamponi M, Szekely N, Appavou MS, Winkler RG, Nauss RE, Johs A, Parks JM, Yi Z, Cheng X, Liang L, Ohl M, Miller SM, Richter D, Gompper G, Smith JC (July 2014). "Structure and dynamics of a compact state of a multidomain protein, the mercuric ion reductase". Biophysical Journal. 107 (2): 393–400. Bibcode:2014BpJ...107..393H. doi:10.1016/j.bpj.2014.06.013. PMC 4104034. PMID 25028881.
- Ledwidge R, Patel B, Dong A, Fiedler D, Falkowski M, Zelikova J, Summers AO, Pai EF, Miller SM (August 2005). "NmerA, the metal binding domain of mercuric ion reductase, removes Hg2+ from proteins, delivers it to the catalytic core, and protects cells under glutathione-depleted conditions". Biochemistry. 44 (34): 11402–16. doi:10.1021/bi050519d. PMID 16114877.
- Engst S, Miller SM (March 1999). "Alternative routes for entry of HgX2 into the active site of mercuric ion reductase depend on the nature of the X ligands". Biochemistry. 38 (12): 3519–29. doi:10.1021/bi982680c. PMID 10090738.