Merkel cell polyomavirus
Merkel cell polyomavirus (MCV or MCPyV) was first described in January 2008 in Pittsburgh, Pennsylvania.[1] It was the first example of a human viral pathogen discovered using unbiased metagenomic next-generation sequencing with a technique called digital transcriptome subtraction.[2] MCV is one of seven currently known human oncoviruses. It is suspected to cause the majority of cases of Merkel cell carcinoma, a rare but aggressive form of skin cancer.[3] Approximately 80% of Merkel cell carcinoma (MCC) tumors have been found to be infected with MCV. MCV appears to be a common—if not universal—infection of older children and adults.[4][5] It is found in respiratory secretions suggesting that it may be transmitted by a respiratory route. But it also can be found shedding from healthy skin, and in gastrointestinal tract tissues and elsewhere, and so its precise mode of transmission remains unknown.[6][7] In addition, recent studies suggest that this virus may latently infect the human sera[8] and peripheral blood mononuclear cells.[9]
| Human polyomavirus 5 | |
|---|---|
| Virus classification | |
| (unranked): | Virus |
| Realm: | Monodnaviria |
| Kingdom: | Shotokuvirae |
| Phylum: | Cossaviricota |
| Class: | Papovaviricetes |
| Order: | Sepolyvirales |
| Family: | Polyomaviridae |
| Genus: | Alphapolyomavirus |
| Species: | Human polyomavirus 5 |
Most MCV viruses found in MCC tumors, however, have at least two mutations that render the virus nontransmissible: 1) The virus is integrated into the host genome and 2) The viral T antigen has truncation mutations that leave the T antigen unable to initiate DNA replication needed to propagate the virus.[10]
Evidence that MCV is the cause for most MCC tumors comes from studies in which T antigen oncoproteins from the virus are inhibited. Knockdown of these viral proteins causes cells from MCV-positive MCC tumors to die whereas there is no effect on cells from tumors that are uninfected with the virus.[11] This indicates that MCV is necessary to maintain the virus-positive tumor cells. Further, clonal pattern of MCV insertions into MCC cell genomes indicates that the virus was present in the Merkel cell before it underwent cancerous transformation. The IARC has recently classified MCV as a class 2A carcinogen.[12]
Classification
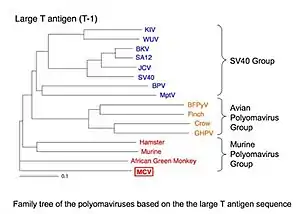
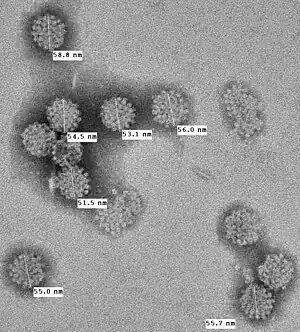
Polyomaviruses are small (~5400 base pair), non-enveloped, double-stranded DNA viruses. MCV is the fifth polyomavirus that infects humans to be discovered. It belongs to the murine polyomavirus group, one of the three main clades of polyomaviruses.[1] (The group is named for murine polyomavirus, the earliest virus of the group to be discovered, and does not imply that MCV is transmitted to humans from rodents.) Although it has been confused with the controversial SV40 virus in some blog postings, it is a distinct virus.
MCV is genetically most closely related to the African green monkey lymphotropic polyomavirus[1] (formerly known as African green monkey lymphotropic papovavirus),[14] which is consistent with MCV coevolving with human primates.
The prototype sequence of MCV has a 5387 base pair double-stranded DNA (dsDNA) genome and encodes characteristic polyomavirus genes from opposite strands including a large T antigen, a small T antigen (LT and sT, respectively, from early strand) and viral capsid proteins VP1 and VP2/3 genes (from late strand) [15] Our Viruses - MCV. MCV T antigen has similar features to the T antigens of other polyomaviruses, which are known oncoproteins, and is expressed in human tumors.[1][10] The T antigen is a spliced gene that forms multiple different proteins depending on the splicing pattern. Both large T and small T oncoproteins are probably needed to transform healthy cells into cancer cells, and they act by targeting tumor suppressor proteins, such as retinoblastoma protein. The LT antigen possesses a helicase motif needed for virus replication that is deleted in MCC tumors. Unlike for other polyomaviruses, MCV sT antigen transforms cells in vitro [16] by activating cap-dependent translation.
MCV also expresses a microRNA (miRNA) known as MCV-miR-M1 from its late strand which bears perfect complementarity to LT and has been shown to negatively regulate LT expression .[17] In addition to its role in regulating MCV LT expression and DNA replication, MCV-miR-M1 has been shown to directly target and downregulate the expression of host cell immune related transcript SP100[18] and its role in the establishment of long-term persistent infection has been demonstrated in vitro.[19]
Viral cause for Merkel cell carcinoma
Merkel cell carcinoma is a highly aggressive type of skin cancer that was first described by Cyril Toker in 1972 as "trabecular tumor of the skin".[20] Based on its origin, the cancer cell type is called a neuroectodermal tumor. Although rare compared with other skin cancers, the incidence of Merkel cell carcinoma in the United States tripled between 1986 and 2001, to around 1400 cases per year.[21]
Merkel cell carcinoma is mainly seen in older individuals.[21] It is known to occur at increased frequency in people with immunodeficiency, including transplant recipients and people with AIDS,[22][23] and this association suggests the possibility that a virus or other infectious agent might be involved in causing the cancer. Kaposi's sarcoma and Burkitt's lymphoma are examples of tumors known to have a viral etiology that occur at increased frequency in immunosuppressed people. Other factors associated with the development of this cancer include exposure to ultraviolet light.[21]
Eight of 10 Merkel cell carcinoma tumors initially tested were found to be infected with MCV.[1] In these tumors, the virus has integrated into the cancer cell genome and can no longer freely replicate. Recent studies from other laboratories have reproduced these findings: in one study 30 of 39 (77%) of Merkel cell tumors were MCV positive;[24] in another study, 45 of 53 (85%) Merkel cell tumors were positive.
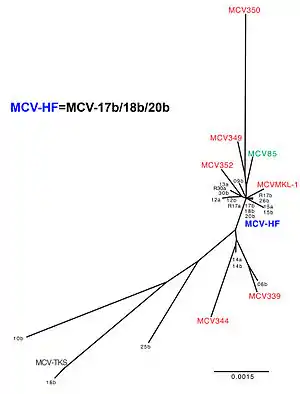
Sequencing of the virus from Merkel cell cancers reveals that it generally has tumor-specific mutations that truncate the MCV T antigen. These mutations (which are not found in native virus obtained from nontumor sites) eliminate the T antigen helicase, preventing the integrated virus from replicating independently from the host cancer cell.[10] The tumor is therefore a "dead-end host" for MCV.[25] Normally, the virus exists as circular episome (or plasmid) within the cell and its DNA is packaged into viral capsids and transmitted to other cells. In tumors, the viral DNA has broken and become integrated into human DNA within the tumor, so that the virus is no longer transmissible. The integrated virus cannot be excised from the host cell and it must replicate as the host cell is replicated. Examination of infected tumors reveals that the majority have a clear monoclonal pattern, indicating that the virus integrated into a single cell before it began its cancerous expansion.[1] For this reason, there is very strong evidence that MCV causes some, but not all, Merkel cell carcinomas. MCV can also be found in healthy tissues from people without Merkel cell carcinoma. A complete MCV genome (MCV-HF) was designed from multiple tumor-type MCV genomes and examined with successful replication capability in vitro.[26] The identical sequences were found in human normal skins.[27] While the precise prevalence of infection is unknown in humans, it is likely that most infections do not cause cancers.[28]
Prevention, diagnosis, and treatment
Persons who have Merkel cell carcinoma with this virus are not infectious to others and no infectious restrictions are warranted. The reasons for this are: 1) the virus in tumors is already mutated and no longer can be transmitted from tumors, and 2) most persons are already naturally exposed to this virus as children and young adults by other asymptomatic carriers.
Based on current data, prevention advice for MCC is similar to other skin cancers, such as avoiding sunburns and unnecessary sun exposure together with use of sun lotion. This may prevent mutations in the virus that increase risk for MCC among those already infected with MCV. Persons with immunosuppression (e.g., AIDS or organ transplant patients) are at higher risk for this cancer and may benefit from periodic skin examinations. Emergence of a painless lump that expands rapidly, especially among persons over age 50 or persons with immunosuppression, warrants examination by a physician. Biopsy of a Merkel cell tumor should readily provide a diagnosis and when caught early, has a good prognosis through standard treatment. At this time there are no vaccines or medications that can prevent MCV infection or prevent emergence of Merkel cell carcinoma.
Detection of the virus is still at a research phase and is generally not available as a clinical test. Detection of viral DNA is performed by PCR or by Southern blot. Caution is needed in interpreting results from PCR since it is prone to false-positive contamination and a substantial fraction of healthy skin samples may harbor low-level infection.[27] Sequencing of the viral genome may determine whether or not tumor-specific mutations are present.
Antibodies have been developed to stain for T antigen in tumor tissues [29] and appear to be specific for MCV-infected tumor cells.[30][31] Blood tests have also been developed[4][5] that show the majority of adults have been previously exposed to MCV and may continue to carry it as an asymptomatic infection.
Treatment guidelines do not differ for Merkel cell carcinoma infected with MCV or without MCV. A recent country-wide study from Finland suggests that MCV-positive tumors have a better prognosis than uninfected tumors[32] (although this has not been found in other studies[25]). If this is confirmed, routine detection of the virus may provide a future benefit for medical guidance. The virus itself is not known to be susceptible to current antiviral medications.
Recent studies reveal that the survivin oncoprotein is activated by MCV large T protein targeting the cellular retinoblastoma protein[33] and that survivin inhibitors can delay tumor progression in animal models. Clinical trials are now being organized to determine whether this has any benefit in humans. The importance of this finding is that a promising rational drug target was uncovered within four years of the initial discovery of the virus and that other new treatments might be rapidly developed now that the cause of the cancer is known. MCV is a target for cell-mediated immune responses, and so important research efforts are being focused on immunologic therapies that may benefit MCC patients.
Discovery and characterization
Yuan Chang and Patrick S. Moore discovered Kaposi's sarcoma-associated herpesvirus by a physical subtraction method in 1994.[34] A virtual subtraction method was developed by Huichen Feng in the lab as a novel high-throughput sequencing technique of digital transcriptome subtraction (DTS)New Pathogen Discovery[2] to search for the presence of a virus in Merkel cell tumors.[1] In this method, all mRNAs from a tumor are converted into cDNAs and sequenced to a depth likely to sequence a viral cDNA if it is present. The sequences are then compared with the human genome and all human sequences are "subtracted" to leave a group of sequences that are most likely nonhuman. When this was performed on four cases of Merkel cell carcinoma, one cDNA was found that was similar to sequences of known polyomaviruses but clearly distinct enough that it could be shown to be a new virus.[1] Genetic sequences from nearly 400,000 mRNAs were analyzed for the study. Once the virus was found, Feng and coworkers quickly determined that infected Merkel cell carcinomas have the virus in an integrated monoclonal pattern and 80% of tissues taken from patients with MCC were positive for the virus. This was quickly confirmed by studies of MCC patients from around the world, including evidence for monoclonal integration of the virus in these tumors.[24][25][35][36]
As a cause for Merkel cell carcinoma
While the original authors conservatively noted that it is "too early to tell" whether MCV is a cause of Merkel cell carcinoma, general scientific opinion now suggests that the virus causes most, but not all Merkel cell tumors. The virus is monoclonally integrated into the tumor when present, indicating that the proto-tumor cell was infected with the virus prior to its cancerous expansion. Mutations in the T antigen render the virus noninfectious, and therefore it is not a passenger virus that infected the tumor after the tumor had already started. Finally, the T antigen oncogene is expressed in all of the tumor cells and when it is inhibited ("knocked down" by RNAi), MCV-positive cells die. Thus, the virus is required for MCV-positive tumors to grow. It is likely that additional host cell mutations act in concert with the integrated virus to actually cause the tumor. Merkel cell carcinoma is associated with exposure to ultraviolet (UV) light and to ionizing radiation, and it is likely that these mutagens increase the rate of mutation in either the virus or the Merkel cell genome, contributing to the risk for cancer after infection.
The reasons why 20% of Merkel cell carcinoma are negative for the virus remain completely unknown but speculations include the possibility that "Merkel cell carcinoma" is actually two or more closely related cancers, only one of which is infected with MCV. Misdiagnosis of this difficult cancer may also account for some of the negative results. Only a very small proportion of people infected with MCV develop the cancer. At this time no test for the presence of the virus is generally available, nor would patients be advised to change their treatment based on knowledge of MCV infection status. MCC patients can be enrolled in research studies, but these are not likely to directly benefit participants.[37] Reducing risk of UV exposure through sun screens is likely to reduce the risk of Merkel cell carcinoma as well as other skin cancers.
Moore has suggested that if his findings are confirmed, information about the virus could lead to a blood test or a vaccine that could improve the management of the disease or aid in prevention, much as the human papillomavirus vaccine can be used to prevent cervical cancer. Chang explained that study of the virus may assist in understanding other human cancers. "Once the virus integrates, it could express an oncoprotein, or it could knock out a gene that suppresses tumor growth. Either way, the results are bound to be interesting."[38][39]
Other associations
Possible associations with cervical carcinoma, cutaneous squamous cell carcinoma, Bowen's disease, basal cell skin carcinoma, extrapulmonary small cell carcinoma, and EGFR mutation-driven non-small cell lung cancer have been reported.[40][41][42][43][44]
References
- Feng H, Shuda M, Chang Y, Moore PS (February 2008). "Clonal integration of a polyomavirus in human Merkel cell carcinoma". Science. 319 (5866): 1096–100. Bibcode:2008Sci...319.1096F. doi:10.1126/science.1152586. PMC 2740911. PMID 18202256.
- Feng H, Taylor JL, Benos PV, Newton R, Waddell K, Lucas SB, Chang Y, Moore PS (October 2007). "Human transcriptome subtraction by using short sequence tags to search for tumor viruses in conjunctival carcinoma". Journal of Virology. 81 (20): 11332–40. doi:10.1128/JVI.00875-07. PMC 2045575. PMID 17686852.
- Rotondo JC, Bononi I, Puozzo A, Govoni M, Foschi V, Lanza G, Gafà R, Gaboriaud P, Touzé FA, Selvatici R, Martini F, Tognon M (July 2017). "Merkel Cell Carcinomas Arising in Autoimmune Disease Affected Patients Treated with Biologic Drugs, Including Anti-TNF". Clinical Cancer Research. 23 (14): 3929–3934. doi:10.1158/1078-0432.CCR-16-2899. hdl:11392/2378829. PMID 28174236.
- Kean JM, Rao S, Wang M, Garcea RL (March 2009). Atwood WJ (ed.). "Seroepidemiology of human polyomaviruses". PLOS Pathogens. 5 (3): e1000363. doi:10.1371/journal.ppat.1000363. PMC 2655709. PMID 19325891.
- Tolstov YL, Pastrana DV, Feng H, Becker JC, Jenkins FJ, Moschos S, Chang Y, Buck CB, Moore PS (September 2009). "Human Merkel cell polyomavirus infection II. MCV is a common human infection that can be detected by conformational capsid epitope immunoassays". International Journal of Cancer. 125 (6): 1250–6. doi:10.1002/ijc.24509. PMC 2747737. PMID 19499548.
- Sloots, Theo P.; Nissen, Michael D.; Whiley, David M.; Lambert, Stephen B.; Bialasiewicz, Seweryn (2009). "Merkel Cell Polyomavirus DNA in Respiratory Specimens from Children and Adults". Emerging Infectious Diseases. 15 (3): 492–4. doi:10.3201/eid1503.081067. PMC 2681122. PMID 19239774.
- Allander, Tobias; Tiveljung-Lindell, Annika; Lindau, Cecilia; Goh, Shan (2009). "Merkel Cell Polyomavirus in Respiratory Tract Secretions". Emerging Infectious Diseases. 15 (3): 489–91. doi:10.3201/eid1503.081206. PMC 2681127. PMID 19239773.
- Mazzoni E, Rotondo JC, Marracino L, Selvatici R, Bononi I, Torreggiani E, Touzé A, Martini F, Tognon MG (2017). "Detection of Merkel Cell Polyomavirus DNA in Serum Samples of Healthy Blood Donors". Front Oncol. 7: 294. doi:10.3389/fonc.2017.00294. PMC 5712532. PMID 29238698.
- Tagliapietra A, Rotondo JC, Bononi I, Mazzoni E, Magagnoli F, Maritati M (2020). "Droplet-digital PCR assay to detect Merkel cell polyomavirus sequences in chorionic villi from spontaneous abortion affected females". J Cell Physiol. 235 (3): 1888–1894. doi:10.1002/jcp.29213. hdl:11392/2409453. PMID 31549405.
- Shuda M, Feng H, Kwun HJ, Rosen ST, Gjoerup O, Moore PS, Chang Y (October 2008). "T antigen mutations are a human tumor-specific signature for Merkel cell polyomavirus". Proceedings of the National Academy of Sciences of the United States of America. 105 (42): 16272–7. Bibcode:2008PNAS..10516272S. doi:10.1073/pnas.0806526105. PMC 2551627. PMID 18812503.
- Houben R, Shuda M, Weinkam R, Schrama D, Feng H, Chang Y, Moore PS, Becker JC (July 2010). "Merkel cell polyomavirus-infected Merkel cell carcinoma cells require expression of viral T antigens". Journal of Virology. 84 (14): 7064–72. doi:10.1128/JVI.02400-09. PMC 2898224. PMID 20444890.
- "Archived copy" (PDF). Archived from the original (PDF) on 2011-10-25. Retrieved 2012-07-17.
{{cite web}}: CS1 maint: archived copy as title (link) - "Addgene: MCV-HF". www.addgene.com. Retrieved 20 April 2018.
- Pawlita M, Clad A, zur Hausen H (May 1985). "Complete DNA sequence of lymphotropic papovavirus: prototype of a new species of the polyomavirus genus". Virology. 143 (1): 196–211. doi:10.1016/0042-6822(85)90108-4. PMID 2998001.
- Theiss JM, Günther T, Alawi M, Neumann F, Tessmer U, Fischer N, Grundhoff A (July 2015). "A Comprehensive Analysis of Replicating Merkel Cell Polyomavirus Genomes Delineates the Viral Transcription Program and Suggests a Role for mcv-miR-M1 in Episomal Persistence". PLOS Pathogens. 11 (7): e1004974. doi:10.1371/journal.ppat.1004974. PMC 4517807. PMID 26218535.
- Shuda M, Kwun HJ, Feng H, Chang Y, Moore PS (September 2011). "Human Merkel cell polyomavirus small T antigen is an oncoprotein targeting the 4E-BP1 translation regulator". The Journal of Clinical Investigation. 121 (9): 3623–34. doi:10.1172/JCI46323. PMC 3163959. PMID 21841310.
- Seo GJ, Chen CJ, Sullivan CS (January 2009). "Merkel cell polyomavirus encodes a microRNA with the ability to autoregulate viral gene expression". Virology. 383 (2): 183–7. doi:10.1016/j.virol.2008.11.001. PMID 19046593.
- Akhbari P, Tobin D, Poterlowicz K, Roberts W, Boyne JR (November 2018). "MCV-miR-M1 Targets the Host-Cell Immune Response Resulting in the Attenuation of Neutrophil Chemotaxis" (PDF). The Journal of Investigative Dermatology. 138 (11): 2343–2354. doi:10.1016/j.jid.2018.03.1527. PMID 29777657.
- Theiss JM, Günther T, Alawi M, Neumann F, Tessmer U, Fischer N, Grundhoff A (July 2015). "A Comprehensive Analysis of Replicating Merkel Cell Polyomavirus Genomes Delineates the Viral Transcription Program and Suggests a Role for mcv-miR-M1 in Episomal Persistence". PLOS Pathogens. 11 (7): e1004974. doi:10.1371/journal.ppat.1004974. PMC 4517807. PMID 26218535.
- Toker C (January 1972). "Trabecular carcinoma of the skin". Archives of Dermatology. 105 (1): 107–10. doi:10.1001/archderm.105.1.107. PMID 5009611.
- Bichakjian CK, Lowe L, Lao CD, Sandler HM, Bradford CR, Johnson TM, Wong SL (July 2007). "Merkel cell carcinoma: critical review with guidelines for multidisciplinary management". Cancer. 110 (1): 1–12. doi:10.1002/cncr.22765. hdl:2027.42/56047. PMID 17520670. S2CID 23833150.
- Williams RH, Morgan MB, Mathieson IM, Rabb H (May 1998). "Merkel cell carcinoma in a renal transplant patient: increased incidence?". Transplantation. 65 (10): 1396–7. doi:10.1097/00007890-199805270-00019. PMID 9625025.
- Engels EA, Frisch M, Goedert JJ, Biggar RJ, Miller RW (February 2002). "Merkel cell carcinoma and HIV infection". Lancet. 359 (9305): 497–8. doi:10.1016/S0140-6736(02)07668-7. PMID 11853800. S2CID 11934339.
- Kassem A, Schöpflin A, Diaz C, Weyers W, Stickeler E, Werner M, Zur Hausen A (July 2008). "Frequent detection of Merkel cell polyomavirus in human Merkel cell carcinomas and identification of a unique deletion in the VP1 gene". Cancer Research. 68 (13): 5009–13. doi:10.1158/0008-5472.CAN-08-0949. PMID 18593898.
- Becker JC, Houben R, Ugurel S, Trefzer U, Pföhler C, Schrama D (January 2009). "MC polyomavirus is frequently present in Merkel cell carcinoma of European patients". The Journal of Investigative Dermatology. 129 (1): 248–50. doi:10.1038/jid.2008.198. PMID 18633441.
- Feng H, Kwun HJ, Liu X, Gjoerup O, Stolz DB, Chang Y, Moore PS (2011). "Cellular and viral factors regulating Merkel cell polyomavirus replication". PLOS ONE. 6 (7): e22468. Bibcode:2011PLoSO...622468F. doi:10.1371/journal.pone.0022468. PMC 3142164. PMID 21799863.
- Schowalter RM, Pastrana DV, Pumphrey KA, Moyer AL, Buck CB (June 2010). "Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin". Cell Host & Microbe. 7 (6): 509–15. doi:10.1016/j.chom.2010.05.006. PMC 2919322. PMID 20542254.
- Viscidi RP, Shah KV (February 2008). "Cancer. A skin cancer virus?". Science. 319 (5866): 1049–50. doi:10.1126/science.1155048. PMID 18292327. S2CID 35809601.
- Shuda M, Arora R, Kwun HJ, Feng H, Sarid R, Fernández-Figueras MT, Tolstov Y, Gjoerup O, Mansukhani MM, Swerdlow SH, Chaudhary PM, Kirkwood JM, Nalesnik MA, Kant JA, Weiss LM, Moore PS, Chang Y (September 2009). "Human Merkel cell polyomavirus infection I. MCV T antigen expression in Merkel cell carcinoma, lymphoid tissues and lymphoid tumors". International Journal of Cancer. 125 (6): 1243–9. doi:10.1002/ijc.24510. PMC 6388400. PMID 19499546.
- Houben R, Schrama D, Alb M, Pföhler C, Trefzer U, Ugurel S, Becker JC (February 2010). "Comparable expression and phosphorylation of the retinoblastoma protein in Merkel cell polyoma virus-positive and negative Merkel cell carcinoma". International Journal of Cancer. 126 (3): 796–8. doi:10.1002/ijc.24790. PMID 19637243. S2CID 9819423.
- Busam KJ, Jungbluth AA, Rekthman N, Coit D, Pulitzer M, Bini J, Arora R, Hanson NC, Tassello JA, Frosina D, Moore P, Chang Y (September 2009). "Merkel cell polyomavirus expression in merkel cell carcinomas and its absence in combined tumors and pulmonary neuroendocrine carcinomas". The American Journal of Surgical Pathology. 33 (9): 1378–85. doi:10.1097/PAS.0b013e3181aa30a5. PMC 2932664. PMID 19609205.
- Sihto H, Kukko H, Koljonen V, Sankila R, Böhling T, Joensuu H (July 2009). "Clinical factors associated with Merkel cell polyomavirus infection in Merkel cell carcinoma". Journal of the National Cancer Institute. 101 (13): 938–45. doi:10.1093/jnci/djp139. PMID 19535775.
- Arora R, Shuda M, Guastafierro A, Feng H, Toptan T, Tolstov Y, Normolle D, Vollmer LL, Vogt A, Dömling A, Brodsky JL, Chang Y, Moore PS (May 2012). "Survivin is a therapeutic target in Merkel cell carcinoma". Science Translational Medicine. 4 (133): 133ra56. doi:10.1126/scitranslmed.3003713. PMC 3726222. PMID 22572880.
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS (December 1994). "Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma". Science. 266 (5192): 1865–9. Bibcode:1994Sci...266.1865C. doi:10.1126/science.7997879. PMID 7997879. S2CID 29977325.
- Sastre-Garau X, Peter M, Avril MF, Laude H, Couturier J, Rozenberg F, Almeida A, Boitier F, Carlotti A, Couturaud B, Dupin N (May 2009). "Merkel cell carcinoma of the skin: pathological and molecular evidence for a causative role of MCV in oncogenesis". The Journal of Pathology. 218 (1): 48–56. doi:10.1002/path.2532. PMID 19291712. S2CID 19709025.
- Buck CB, Lowy DR (January 2009). "Getting stronger: the relationship between a newly identified virus and Merkel cell carcinoma". The Journal of Investigative Dermatology. 129 (1): 9–11. doi:10.1038/jid.2008.302. PMC 3401601. PMID 19078983.
- "New Pathogen Discovery:Frequently Asked Questions". KSHV laboratory, molecular virology program, University of Pittsburgh Cancer Institute. Archived from the original on 2008-04-22. Retrieved 2008-04-13.
- "Newly discovered virus linked to deadly skin cancer". University of Pittsburgh Medical Center News Bureau. 2007-01-17. Retrieved 10 July 2020.
- Allison Gandey (2008-01-18). "newly discovered virus linked to neuroendocrine cancer of the skin". MedScape Medical News.
- Imajoh M, Hashida Y, Nemoto Y, Oguri H, Maeda N, Furihata M, Fukaya T, Daibata M (August 2012). "Detection of Merkel cell polyomavirus in cervical squamous cell carcinomas and adenocarcinomas from Japanese patients". Virology Journal. 9 (1): 154. doi:10.1186/1743-422x-9-154. PMC 3545865. PMID 22876976.
- Murakami M, Imajoh M, Ikawa T, Nakajima H, Kamioka M, Nemoto Y, Ujihara T, Uchiyama J, Matsuzaki S, Sano S, Daibata M (January 2011). "Presence of Merkel cell polyomavirus in Japanese cutaneous squamous cell carcinoma". Journal of Clinical Virology. 50 (1): 37–41. doi:10.1016/j.jcv.2010.09.013. PMID 20965777.
- Zur Hausen A (December 2009). "[Merkel cell polyomavirus in the pathogenesis of non-melanoma skin cancer]". Der Pathologe. 30 Suppl 2 (Suppl 2): 217–20. doi:10.1007/s00292-009-1222-4. PMID 19921198.
- Hourdequin KC, Lefferts JA, Brennick JB, Ernstoff MS, Tsongalis GJ, Pipas JM (October 2013). "Merkel cell polyomavirus and extrapulmonary small cell carcinoma". Oncology Letters. 6 (4): 1049–1052. doi:10.3892/ol.2013.1483. PMC 3796380. PMID 24137462.
- Xu S, Jiang J, Yu X, Sheng D, Zhu T, Jin M (March 2014). "Association of Merkel cell polyomavirus infection with EGFR mutation status in Chinese non-small cell lung cancer patients". Lung Cancer. 83 (3): 341–6. doi:10.1016/j.lungcan.2014.01.002. PMID 24485957.