Metasilicate
Metasilicates are silicates containing ions of empirical formula SiO2−
3. Common stoichiometries include MI
2SiO3 and MIISiO3. Metasilicates can be cyclic, usually the hexamer (SiO3)12−6 or chains (SiO3)n2−.[1]
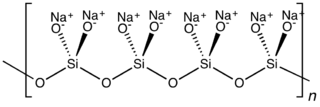
Idealized structure of sodium metasilicate.
Common compounds containing metasilicate anion are:

Pyroxene (diopside) is a mineral classified as a metasilicate.
References
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.