Methyl hydroperoxide
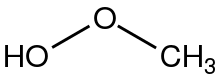
Methyl hydroperoxide is the organic compound with the formula CH3OOH. It is a volaltile colorless liquid. In addition to being of theoretical interest as the simplest organic hydroperoxide, methyl hydroperoxide is an intermediate in the oxidation of methane in the atmosphere.[1] When condensed or in concentrated form methyl hydroperoxide is rather explosive, unlike tertiary hydroperoxides such as tert-butylhydroperoxide.[2] Its laboratory preparation was first reported in 1929.[3]
 | |
| Names | |
|---|---|
| IUPAC name
Methaneperoxol | |
| Other names
Hydroperoxide, methyl Methane hydroperoxide Methyl hydrogen peroxide Hydroperoxymethane | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| CH4O2 | |
| Molar mass | 48.041 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.9967 g/cm3 at 15°C |
| Melting point | <25 °C |
| Boiling point | 46 °C (115 °F; 319 K) |
| Miscible in water and diethyl ether | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
explosive |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- Logan, Jennifer A.; Prather, Michael J.; Wofsy, Steven C.; McElroy, Michael B. (1981). "Tropospheric chemistry: A global perspective". Journal of Geophysical Research. 86 (C8): 7210. Bibcode:1981JGR....86.7210L. doi:10.1029/JC086iC08p07210.
- Roger A. Sheldon (1983). Patai, Saul (ed.). Syntheses and Uses of Hydroperoxides and Dialkylperoxides. PATAI'S Chemistry of Functional Groups. John Wiley & Sons. doi:10.1002/9780470771730.ch6.
- Rieche, Alfred; Hitz, Fritz (1929). "Über Monomethyl-hydroperoxyd)". Berichte der Deutschen Chemischen Gesellschaft. 62 (8): 2458–2474. doi:10.1002/cber.19290620888.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.